Suggestions
Use up and down arrows to review and enter to select.Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews April 25, 2024 April 18, 2024
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

As a student, you are familiar with pressure. Work needs to be done, and there is always a limited time to do it. The less time there is, the more pressure you feel. Gaseous pressure works in much the same way. A force acts on a limited area to give pressure. If the area shrinks (you have less time), something has got to give: either the force shrinks (you take on less work) or the pressure rises.
Pressure is defined mathematically as force divided by the area over which the force acts:
Pressure = 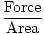 |
| Unit | Relationship | ||
| Pascal (Pa) |
|
||
| Torr |
|
||
| 1 mm Hg at 0o C |
|
||
| atmosphere (atm) |
|
||
| Bar |
|
||
| 1 Pound per Square Inch (psi) |
|
If you've ever checked your tires' pressure, you've probably encountered pounds per square inch, or psi. The other units are less familiar: they arose because gravity exerts a downward force on the atmosphere, which consequently exerts a pressure on the Earth's surface and whatever else happens to be there. On a calm day at sea level, the force gravity exerts is 1.01325×105 N per 1 m2. Since pressure = force / area, this gives a pressure of 1.01325×105 Pa. One standard atmosphere (atm) is defined as exactly 1.01325×105 Pa.
So how did we figure out that standard atmospheric pressure is 1.01325×105 Pa in the first place? Atmospheric pressure was first measured with a
barometer. A barometer consists of a large dish and a long glass tube that
is sealed at one end. The tube and dish are filled with mercury (HG) or some
other liquid, and the tube is inverted into the dish. If all this is done
without any air entering the tube, a column of liquid will remain above the
dish.
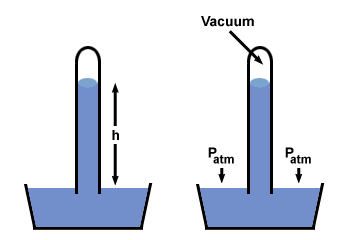
When the tube full of mercury is inverted in the dish, the mercury level will drop. It will continue to drop until the pressure generated by the column's weight equals the atmospheric pressure. Since we know the column's height h, the density of mercury ρ, and the acceleration due to gravity g (9.81m s-2), we can calculate the atmospheric pressure P.
baroeq
| P = ghρ |
Students are often given a non-Hg barometer or conditions where g does not equal 9.8 m/s2. Don't let these types of questions phase you; realize which variables are changing, convert everything down to SI units, and plug them all in to @@Equation@@. There are examples in the problem section.
Please wait while we process your payment

