Suggestions
Use up and down arrows to review and enter to select.Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews May 1, 2024 April 24, 2024
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Problem :
| 4NH3(g) + 6NO(g)→5N2(g) + 6H2O(g) |
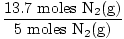 ×4 moles NH3(g) = 10.96 moles NH3(g) ×4 moles NH3(g) = 10.96 moles NH3(g) | |||
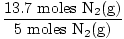 ×6 moles NO(g) = 16.44 moles NO(g) ×6 moles NO(g) = 16.44 moles NO(g) |
Problem : What is the mass of 2 moles of H2S?
GFM of H = 1
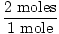 ×34 grams = 68 grams ×34 grams = 68 grams |
Problem : 2Al +3Cl2→2AlCl3
When 80 grams of aluminum is reacted with excess chlorine gas, how many
formula units of AlCl3 are produced?
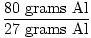 ×1 mole Al = 2.96 moles Al ×1 mole Al = 2.96 moles Al |
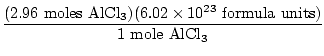 = 1.78×1025 = 1.78×1025 |
Problem : Sb2S3(s) + 3Fe(s)→2Sb(s) +3FeS(s)
If 3.87×1023 particles of
Sb2S3(s) are
reacted with excess Fe(s), what mass of FeS(s) is produced?
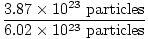 ×1 mole Sb2S3(s) = 0.643 moles Sb2S3(s) ×1 mole Sb2S3(s) = 0.643 moles Sb2S3(s) | |||
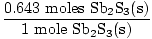 ×3 moles FeS(s) = 1.93 moles FeS(s) ×3 moles FeS(s) = 1.93 moles FeS(s) | |||
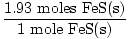 ×88 grams = 170 grams ×88 grams = 170 grams |
Problem : CO(g) +2H2(g)→CH3OH(g)
At STP, what volume of H2(g) is needed to react completely
with 8.02×1023 molecules of CO(g)?
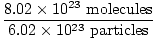 ×1 mole CO(g) = 1.33 moles CO(g) ×1 mole CO(g) = 1.33 moles CO(g) | |||
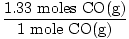 ×2 moles H2(g) = 2.66 moles H2(g) ×2 moles H2(g) = 2.66 moles H2(g) | |||
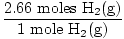 ×22.4 L = 59.7 L ×22.4 L = 59.7 L |
Please wait while we process your payment

