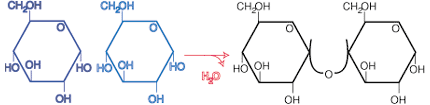
Lactose
Lactose is a disaccharide formed through the condensation of glucose and galactose. The bond formed between the two monosaccharides is called a beta glycosidic bond (). The alpha glycosidic bond, found in sucrose and maltose, differs from the beta glycosidic bond only in the angle of formation between the two sugars. Unfortunately, unlike alpha glycosidic bonds, beta-glycosidic bonds are unable to be digested by some people. Therefore, many people are lactose intolerant and suffer from intestinal cramping and bloating due to the incomplete digestion of the substance.
Sucrose
Sucrose is found in common table sugar and is composed of glucose and fructose
linked via a 1-2 alpha glycosidic bond.
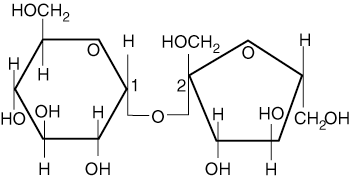
Maltose
Maltose is the final disaccharide and consists of two glucose molecules joined by an alpha glycosidic bond. Maltose is an interesting compound because of its use in alcohol production. Through a process called fermentation, glucose, maltose and other sugars are converted to ethanol by yeast cells in the absence of oxygen. Through an analogous process, muscle cells convert glucose into lactic acid to obtain energy while the body operates under anaerobic conditions. Although maltose is uncommon in nature, it can be formed through the breakdown of starch by the enzymes of the mouth.
Oligosaccharides and Polysaccharides
Carbohydrates that contain more than two simple sugars are called oligosaccharides or polysaccharides, depending upon the length of the structure. Oligosaccharides usually have between three and ten sugar units while polysaccharides can have more than three thousand units. These large structures are responsible for the storage of glucose and other sugars in plants and animals.
Oligosaccharides
Important oligosaccharides are raffinose and stachyose. Composed of repeating units of galactose, glucose and fructose, these oligosaccharides are of nutritional importance because they are found in beans and legumes. Because of their unique glycosidic bonds, raffinose and stachyose cannot be broken down into their simple sugars. Therefore, they cannot be absorbed by the small intestine and are often metabolized by bacteria in the large intestine to form unwanted gaseous byproducts. Commercial enzyme preparations such as Beano can be consumed before a meal rich in beans and legumes in order to aid the small intestine in the breakdown of these oligosaccharides.
Polysaccharides
Polysaccharides or complex carbohydrates are usually monomers and consist of thousands of repeating glucose units. Naturally, they allow for the storage of large quantities of glucose. Starch is the major storage form of carbohydrate in plants and has two different types: amylose and amylopectin. Although digestible alpha glycoisidic bonds link both types of starch, each type is unique in their branching of glucose. While amylose is a straight chain polymer, amylopectin is highly branched. These differences account for the fact that amylopectin can form stable starch gels which are able to retain water while amylose is unable to do so. Therefore, amylopectin is often used by manufacturers to produce many different kinds of thick sauces and gravies. Sources of starch include potatoes, beans, bread, pasta, rice and other bread products.


 payment page
payment page



