Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Cellular Energy Sources
The goal of cellular respiration and metabolism in animals and plants is, ultimately, the conversion of one type of energy source to another. Presumably, the original energy source comes in a form that cannot be immediately used to support cellular activities. For humans, our external energy sources are the foods we eat. Once we ingest and digest the food, our cells metabolic processes convert the energy contained within the food into a form of energy that can function in our cells. These constant conversions are what allow us to perform our day-to-day activities.
Since energy is the ultimate goal of metabolism, it will be helpful to understand what these various external and internal energy sources really are. As we have mentioned, food is the external energy source for humans. Different foods are composed primarily of one of the following three macromolecules: carbohydrates (breads and pastas), lipids (fats and oils), or proteins (meats and beans). During digestion of food, when the food is first broken down internally, these large molecules are broken into subunits. Depending on their type, subunits can be metabolized in different ways and then used as internal energy sources.
The distinct means of metabolizing specific subunits all have the same goal, the production of the primary cellular energy source: adenosine triphosphate.
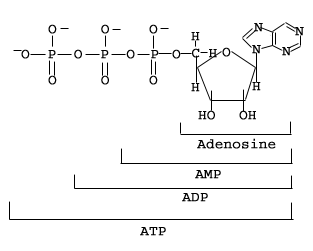
As you can see in the figure above, ATP contains three phosphate groups. These groups are primarily responsible for ATP's role as an energy source. During metabolic reactions, these phosphate groups can be transferred from ATP to yield either adenosine diphosphate (ADP) or adenosine monophosphate (AMP).
ATP -> ADP + P + energy, orThe release of one or more phosphate groups is energetically favorable: the reaction produces energy. ATP can also undergo a reaction with water to yield ADP or AMP to release energy. The cell can use the energy produced from the breakdown of ATP for whatever purpose is necessary. Often, the energetically favorable breakdown of ATP is often coupled with another, energetically unfavorable reaction that is designed to drive the first reaction forward through the synthesis of additional ATP.
ATP -> AMP + 2P + energy
ATP synthesis is almost exactly opposite to the process by which ATP is broken down to produce energy: phosphate groups are brought in contact with either ADP or AMP. While this process is not as favorable, it is able to occur with the energy derived from metabolizing foods. In addition to ATP, there are a number of other reactive molecules that are involved in the production of cellular energy. These are called coenzymes and their role is to help transfer other chemical groups like hydrogens. Coenzymes work in conjunction with metabolic enzymes to drive metabolic reactions. Among these are nicotinamide adenine dinucleotide (NADH) and acetyl coenzyme A. We will discuss the specific roles of both these molecules more in following sections.
Please wait while we process your payment





