Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

The Covalent Bond
A covalent bond represents a shared electron pair between nuclei. The Stability of covalent bonds is due to the build-up of electron density between the nuclei. Using Coulomb's law (discussed in Ionic Bonding), you should note that it is more stable for electrons to be shared between nuclei than to be near only one nucleus. Also, by sharing electron pairs nuclei can achieve octets of electrons in their valence shells, which leads to greater stability.
To keep track of the number and location of valence electrons in an atom or molecule, G. N. Lewis developed Lewis structures. A Lewis structure only counts valence electrons because these are the only ones involved in bonding. To calculate the number of valence electrons, write out the electron configuration of the atom and count up the number of electrons in the highest principle quantum number. The number of valence electrons for neutral atoms equals the group number from the periodic table. Each valence electron is represented by a dot next to the symbol for the atom. Because atoms strive to achieve a full octet of electrons, we place two electrons on each of the four sides of the atomic symbol. Some examples of Lewis structures for atoms are shown in .
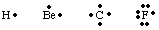
We can create bonds by having two atoms come together to share an electron pair. A bonding pair of electrons is distinguished from a non-bonding pair by using a line between the two atoms to represent a bond, as in the figure below. A lone pair is what we call two non-bonding electrons localized on a particular atom.
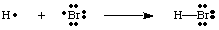
You should note that each atom in the H-Br molecule has a full valence shell. Both the hydrogen and the bromine can count the two electrons in the bond as its own because the electrons are shared between both atoms. Hydrogen needs only two electrons to fill its valence, which it gets through the covalent bond. The bromine has an octet because it has two electrons from the H-Br bond and six more electrons, two in each lone pair on Br.
The deadly gas carbon monoxide, CO, provides an interesting example of how to draw Lewis structures. Carbon has four electrons and oxygen has six. If only one bond were to be formed between C and O, carbon would have five electrons and oxygen 7.
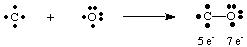
A single bond here does not lead to an octet on either atom. Therefore, we propose that more than one bond can be formed between carbon and oxygen so that we can give each atom an octet of electrons. To complete the carbon and oxygen octets in CO, we must employ a triple bond, denoted by three lines joining the C and O atoms as shown in . A triple bond means that there are six electrons shared between carbon and oxygen. Such multiple bonds must be employed to explain the bonding in many molecules. However, only single, double, and triple bonds are commonly encountered.
Please wait while we process your payment





