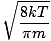-
Boltzmann constant
A constant, k, involved in the equation for average velocity. k = 1.38×10-23 J/K
-
Diffusion
Diffusion is the spread of one substance through another.
-
Effusion
Effusion is the rate at which a gas passes through a small hole into a vacuum.
-
Kinetic energy
Ek = 1/2mv2
-
Kinetic molecular theory
A theory that models the interaction between individual gas molecules. See the summary for more details.
-
Maxwell-Boltzmann speed distribution
The distibution attained when molecule speed is set against the number of molecules sharing that speed.
-
Mean free path
The mean distance a molecule travels before it impacts another molecule; given the huge number of collisions in a gas, the mean free path is vastly smaller than any typical room or container. The equation for mean free path:
λ = 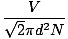
-
mp
The most probable velocity at which the most molecules in a gas travel. The formula for most probable velocity is:
vp = 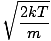 =
= 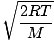
-
Root mean square velocity
An equation to measure the typical velocity of molecules in a gas.
vrms = 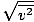
= 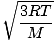
= 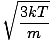
Terms
Formulae
Average velocity 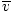 |
|
| Kinetic energy | Ek = 1/2mv2 |
| Mean free path |
|
| Formule for most probable velocity |
|
| Formula for root mean square velocity |
|










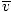 =
= 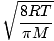 =
=