The van der Waals equation corrects for the volume of, and attractive forces
between, gas molecules:
(P + 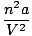 )(V - nb) = nRT )(V - nb) = nRT |
|
There are two corrective factors in van der Waals equation. The first,
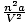
, alters the pressure in the ideal gas equation. It accounts
for the intermolecular attractive forces between gas molecules. The
magnitude of
a is indicative of the strength of the intermolecular attractive
force.
a has units of
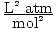
.
The factor - nb accounts for the volume occupied by the gas molecules. b has
units of L/mol. Since b corresponds to the total volume per mole occupied by
gas molecules, it closely corresponds to the volume per mole of the liquid
state, whose molecules are closely layered. b is generally much smaller in
magnitude than a. The values of a and b generally increase with the size and
complexity of the molecule.
Unfortunately, the values of a and b must be experimentally determined. By
now you should be at ease manipulating the ideal gas law. Van der Waals
equation isn't much different. The only trick is remembering the corrective
factors.
Even if you forget the equations of the corrective factors, don't panic. You
can deduce them from the values of a and b, which the question will most
likely give you since they are experimental. More importantly, the question
must give you the units of a and b. If you know the units of a and
b, you should be able to work backwards to pressure or volume. Note that the
units of a do not include volume, and that the units of b do not include
pressure. So you shouldn't get them confused. Try working backwards from the
units of a and b to their full corrective factors.