Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews July 1, 2025 June 24, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Mechanisms of Chemical Reactions
Mechanisms describe in a stepwise manner the exact collisions and events that are required for the conversion of reactants into products. Mechanisms achieve that goal by breaking up the overall balanced chemical equation into a series of elementary steps. An elementary step is written to mean a single collision or molecular vibration that results in a chemical reaction. The following picture of an elementary step shows a single collision between water and boron trifluoride:
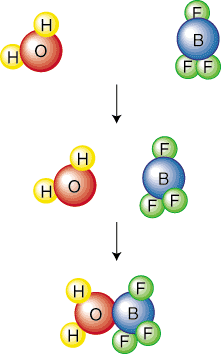
The molecularity of an elementary step describes the number of reactive partners in the elementary step. For example, the above elementary step is called bimolecular because two molecules collide. Commonly, elementary steps are mono-, bi-, or termolecular. The probability of four molecules colliding at exactly the same place and time is so small that we can safely assume that no reaction will ever be tetramolecular. Because take up a large amount of space, we will represent elementary steps in this SparkNote as normal reactions with molecular formula line equations. You will know from the context (i.e. talking about the steps of a mechanism) whether the reaction is an elementary step or an overall reaction.
To better understand mechanisms, let's consider the following mechanism for the decomposition of ozone, O3:
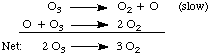
The above mechanism exhibits a property of all mechanisms: it is a series of elementary steps whose sum is the overall balanced reaction. Note the presence of the oxygen atom, O, intermediate in the above equation. It is an intermediate because it is both created and destroyed in the mechanism and does not appear in the net equation.
Another property of mechanisms is that they must predict the experimentally determined rate law. To calculate the rate law from a mechanism you need to first know the rate limiting step. The rate limiting step determines the rate of the reaction because it is the slowest step. You can rationalize that a reaction can only go so fast as its slowest step by thinking about what happens when you encounter an accident on the highway that closes all but one lane. You may have been able to race along at 65 m.p.h. (depending on your state's laws) before you reached the lane closure but the slow passage of cars past the accident limits your rate. You can only go as fast through that one lane as the slowest car in front of you.
In the above , the first reaction is labeled as "slow". This reaction is the rate determining step because it is the slowest step. As we have stated, that means that the rate of the overall reaction is equal to the rate of the rate determining step. The rate of an elementary step is the rate constant for that step multiplied by the concentrations of the reactants raised to their stoichiometric powers. Note that this rule only applies for elementary steps. The rate of an overall reaction is NOT the product of the concentrations of the reactants raised to their stoichiometric powers. The rate law for the first elementary step in the is rate = k [O3]. Because this step is the rate determining step, the rate law is also the rate law for the overall reaction. Using similar techniques we can calculate the rate law predicted by any mechanism. We then check the predicted rate law against the experimentally determined rate law to test the validity of the proposed mechanism.
Please wait while we process your payment

