Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Resonance
When drawing Lewis structures, sometimes you will find that there are many ways to place double bonds and lone pairs about a given framework of atoms. How does we decide whether one or another placement is correct? The answer, as it turns out, is neither and both. The actual arrangement of electrons in a given molecule is a weighted average of all the valid Lewis structures that can be drawn for that given atomic connectivity. The "real" molecule, the one that actually exists in the world, is said to be a resonance hybrid of all its contributing Lewis structures. Each Lewis structure that contributes to the resonance hybrid is a resonance structure.
The classical example of resonance is benzene, C6H6. Two good Lewis structures for benzene exist that differ only in their placement of double bonds. If either structure were correct, benzene would consist of alternating long single bonds and short double bonds. However, it has been determined experimentally that all six bonds on the ring are identical. The natural interpretation is that the three double bonds are distributed evenly around the ring, so that each bond has a bond order of one and a half.
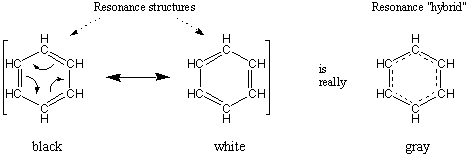
A double headed arrow is placed between resonance structures to denote them as such. In addition, sometimes we place all the resonance contributors within brackets for clarity.
It's important to remember that although the molecule described by resonance has characteristics of all its resonance contributors, it is fully neither one. For instance, the color gray might be described as being a resonance hybrid of white and black. And although gray takes on characteristics of both black and white, it would be incorrect to describe gray as being black or white.
Sometimes double-headed arrows are used to denote how one resonance structure
can be derived from another via the flow of electrons. This curved-arrow
formalism is a very useful bookkeeping tool that allows us to keep track of
the movement of pairs of electrons during reactions. The arrows are drawn from
the source of the electron motion, which can be a bonded electron pair or a lone
pair, to the destination of the electrons, typically an atom or a place between
two atoms. The figure below illustrates correct and incorrect usages of the
curved-arrow formalism. We will see that it is a very useful tool for
describing reaction mechanisms, the step-by-step processes by which
reactions occur. Such exercises are affectionately referred to as "arrow
pushing".

Resonance is such an important concept to master early on in your organic chemistry education that it's worthwhile to clear up two potential misunderstandings. Resonance and equilibrium, and resonance and isomerism are often confused.
Please wait while we process your payment





