Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Valence Bond Theory
So far we've presented a straightforward view of covalent bonding as the sharing of electrons between two atoms. However, we have yet to answer questions such as these: How are electrons shared? What orbitals do shared electrons reside in? Can we say anything about the energies of these shared electrons? Our task now is to extend the orbital scheme that we've developed for atoms to describe bonding in molecules.
Valence bond theory (VB) is a straightforward extension of Lewis
structures. Valence bond theory says that electrons in a covalent bond
reside in a region that is the overlap of individual atomic
orbitals. For example, the covalent bond in molecular hydrogen can be
thought of as result of the overlap of two hydrogen 1s orbitals.

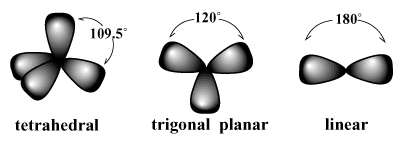
In order to understand the limitations of valence bond theory, first we must digress to discuss molecular geometry, which is the spatial arrangement of covalent bonds around an atom. A very simple and intuitive approach, the Valence Shell Electron Pair Repulsion (VSEPR) model, is used to explain molecular geometry. VSEPR states that electron pairs tend to arrange themselves around an atom in such a way that repulsions between pairs are minimized. /PARGRAPH For instance, VSEPR predicts that carbon, which has a valence of four, should have a tetrahedral geometry. This is the observed geometry of methane (CH4). In such an arrangement, each bond about carbon points to the vertices of an imaginary tetrahedron, with bond angles of 109.5 degrees, which is the largest bond angle that can be attained between all four bonding pairs at once. Similarly, the best arrangement for three electron pairs is a trigonal planar geometry with bond angles of 120 degrees. The best arrangement for two pairs is a linear geometry with a bond angle of 180 degrees.
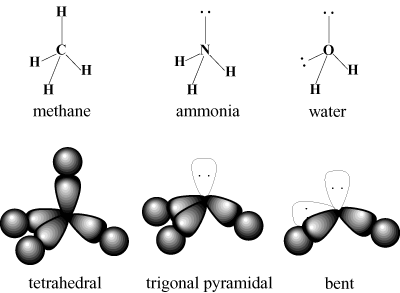
The VSEPR scheme includes lone pairs as well as bonded pairs. Since lone pairs are closer to the atom, they actually take up slightly more space then bonded pairs. However, lone pairs are "invisible" as far as the geometry of the atom is concerned. For instance, ammonia (CH3) has three C-H bonded pairs and one lone pair. These four electrons will, like methane, occupy a tetrahedral arrangement. Since lone pairs take more space, the H-N-H bond angle is reduced from 109.5 degrees to about 107 degrees. The geometry of ammonia is trigonal pyramidal rather than tetrahedral since the lone pair is not included. By similar reasoning, water has a bent geometry with a bond angle of about 105 degrees.
Note that multiple bonds don't affect the VSEPR scheme. A double or triple bond is considered no more repulsive than a single bond.
The Valence Bond model runs into problems as soon as we try to take
molecular geometries into account. The tetrahedral geometry of methane
is clearly impossible if carbon uses its 2s and 2p orbitals to form the C-H
bonds, which should yield bond angles of 90 degrees.

Please wait while we process your payment





