Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Introduction to Organic Molecules
As the term "organic" implies, organic chemistry had its origins in the study of natural compounds extracted from living organisms. It was believed that these compounds contained a "vital force" that was responsible for life processes. This theory of "vitalism" held that organic compounds were somehow beyond the grasp of experimental science. Vitalism was disproved when Friederich Wohler accidentally created the organic compound urea by heating ammonium cyanate, which was classified as inorganic.
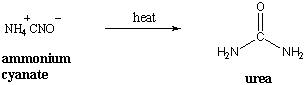
Since then, the definition of organic chemistry has been expanded to the study of compounds that contain carbon. Carbon is special for several reasons:
Indeed, almost all molecules of biological importance are built on such carbon frameworks. However, carbon-containing molecules are useful not only to biological systems but in industries as diverse as pharmaceutical medicine, food, clothing, communications, and heavy industry. The task of organic chemists is two-fold: to study organic molecules from a theoretical perspective and to learn new strategies for the synthesis and application of complex molecules in these industries.
The simplest organic molecules are hydrocarbons, compounds that contain only carbon and hydrogen. Two broad classes of hydrocarbons are aliphatic hydrocarbons and aromatic hydrocarbons. Aromatic hydrocarbons contain benzene-like structures, and we'll see in upcoming chapters that such compounds exhibit special chemistry. Aliphatic hydrocarbons don't contain benzene rings and typically consist of carbon chains connected by single, double, and triple bonds. Aliphatic hydrocarbons that contain only single bonds are alkanes. Hydrocarbons that contain double bonds are alkenes and those with triple bonds are alkynes.
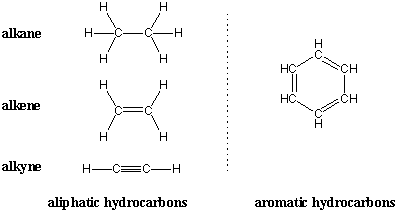
We begin our study of organic molecules with alkanes, chains of carbon atoms held by single bonds. Even simple alkanes exhibit the structural diversity mentioned previously: an alkane can be unbranched or branched, and it can also loop back on itself to form a cyclic alkane. Cyclic alkanes will be considered separately later in this chapter. All acyclic alkanes (unbranched and branched) have the characteristic molecular formula CnH(2n + 2), where n is the number of carbon atoms in the chain. gives the molecular formulas and Lewis structure for the unbranched, or n-alkanes (n stands for normal). Notice that each n-alkane differs from the next one in the series by a (-CH2-), or methylene group.
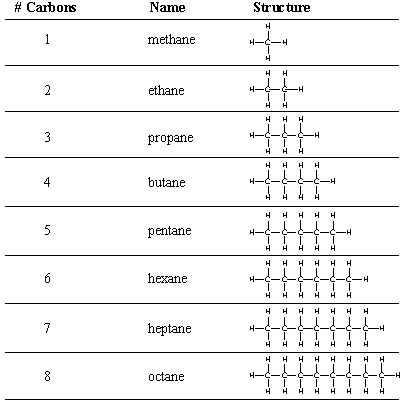
As you can see from the structures in the figure above, drawing out full Lewis structures even for simple organic molecules can be quite tedious. Several shorthand notations are used by organic chemists to designate molecules more complex than methane and ethane. A condensed structural formula omits the single bonds to hydrogens. Sometimes even the carbon-carbon bonds are omitted. For example, hexane can be drawn using the following condensed structures.
Please wait while we process your payment





