Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Introduction to Cycloalkanes
Carbocycles are organic molecules that contain one or more rings, chains of atoms that loop back on themselves. The simplest cyclic molecules are the cycloalkanes, which have molecular formulas CnH2n. Cycloalkanes are named after their corresponding linear alkanes with the prefix -cyclo. Cycloalkanes can be drawn as regular polygons using line-angle representations.
Substituted cycloalkanes are named similarly to linear alkanes, as the following examples illustrate. The positions on the ring are numbered in such a way that substituents receive the lowest possible numberings. Since all positions on the ring are equivalent except for the attachment of substituents, numbers are only indicated in the name of the compound when more than one substituent is present.
Like alkenes, cycloalkanes are capable of cis-trans isomerism. A
cycloalkane has two distinct faces, and any substituent on a ring lies
toward one of two faces. When two substituents on a ring point to the same
face, they are cis. When the two substituents point to opposite
faces, they are trans. Like the cases of cis-trans
isomerism in
alkenes, these isomers have the same atomic connectivities but differ in
their spatial arrangement of atoms. Hence, they are
stereoisomers.

The heat of formation of a molecule is the energy change that occurs when a molecule is assembled from its component atoms. Heats of formations typically have negative signs, indicating that the molecule is more stable than its component atoms. First, consider the heats of formations of the n-alkanes, which advance regularly by -4.95 kcal/mol for each increase in chain length. Since each unbranched alkane differs from the next in the series by a methylene (-CH2-) group, we infer that - 4.95 is the heat of formation associated with each methylene group. The cycloalkanes, which have molecular formulas of (CH2)n, consist of methylene groups arranged in a ring. Hence we might expect the heat of formation of any n carbon cycloalkane to be n times -4.95.
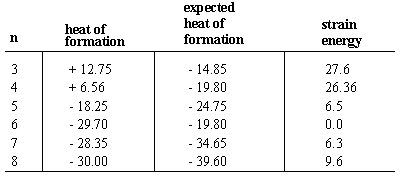
In every case except cyclohexane, the actual heat of formation is less negative than the predicted value. That is, cycloalkanes are less stable than their straight-chain counterparts due to ring strain, unfavorable energetics caused by ring formation. Rings strains can be calculated from the difference between actual and expected heats of formation. Both cyclopropane and cyclobutane have large ring strains of 27 kcal/mol and 26 kcal/mol, respectively. Cyclopentane has much less ring strain at 6.5 kcal/mol. Cyclohexane is the only cycloalkane that has no ring strain. Cycloheptane and higher cycloalkanes tend to have modest amounts of ring strain (although strain diminishes for very large rings, where the length of the ring allows atoms to arrange themselves in low-energy conformations).
Bridged ring systems are particularly rigid due to ring strain. In a bridged system, bridgehead carbons are the points at which the two cycles meet. These carbons are nearly always singly bonded, or sp3-hybridized. Forming a Π bond would require sp2 hybridization and a trigonal planar geometry that would be terribly strained in the context of the ring constraints. This concept is summarized by Bredt's Rule: No bridgehead alkenes.
Please wait while we process your payment





