Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Conformations of Ethane
In the last chapter, we saw that it was possible to describe the complete 3-dimensional shape of methane by specifying its bond angles and bond lengths. Ethane, which is comprised of two methyl groups attached to each other, has properties very similar to those of methane. However, the complete 3-dimensional shape of ethane cannot be specified by these bond lengths and bond angles alone because ethane can internally rotate about its C-C bond.
To understand why ethane has this extra degree of freedom, consider the cylindrically symmetric nature of $\sigma$ bonds. The $\sigma$ bond can maintain a full degree of overlap while its two ends rotate. Hence, the energetic barrier to rotation about sigma bonds is generally very low. Unlike $\pi$ bonds in alkenes, the C-C sigma bond does not hold the two methyl groups in fixed positions relative to one another. The different spatial arrangements formed by rotations about a single bond are called conformations or conformers.
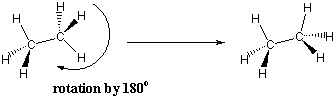
Several methods are used by organic chemists to help them visualize the conformations of molecules. One of these methods uses wedges to denote bonds that are extending out from the plane of the page toward the reader and dashes to indicate bonds that are going into the plane of the page away from the reader. This notation is frequently used to represent the tetrahedral geometry of $sp^3$-hydridized carbons.
A Newman projection can be used to specify the conformation of a particular bond with clarity and detail. A Newman projection represents the head-on look down the bond of interest. The circle in the Newman projection represents the atom in front of the bond, and the lines radiating from the center are the bonds of that atom. The bonds of the rear atom emerge from the sides of the circle.
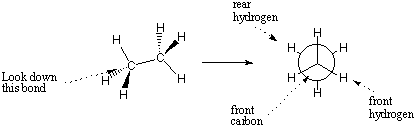
Newman projections can be characterized by the angles formed between bonds on the front atom and bonds on the rear atom. Such angles are called dihedral angles. The full 3D shape of any molecule can be described by its bond lengths, bond angles, and dihedral angles.
While there are an infinite number of conformations about any sigma bond, in ethane two particular conformers are noteworthy and have special names. In the eclipsed conformation, the C-H bonds on the front and back carbons are aligned with each other with dihedral angles of 0 degrees. In the staggered conformation, the C-H bonds on the rear carbon lie between those on the front carbon with dihedral angles of 60 degrees.
Please wait while we process your payment





