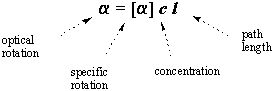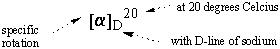Chiral Interactions
Think back to our first examples of chiral objects. We said that your left hand would have a hard time fitting into a left-handed glove. Yet at the same time, either hand could pick up a cup with the same facility. It is apparent that chiral objects of either handedness interact equally well with some objects, but not others. Where does this difference arise? It turns out that chiral objects of opposite handedness interact with achiral objects equally well. They do not interact equally well with chiral objects. For instance, a glove is a chiral object, whereas a cup is not. For a more vivid example, consider what happens during a handshake: a right hand can only shake a right hand, and not a left one. The functions of your hands differ because their interacting environments (in this case the hands you are shaking) are themselves chiral.
Optical Activity
Generally, enantiomers have identical physical properties, such as densities, boiling points, melting points, and refractive indices. This poses a problem for experimentalchemists who are working with chiral compounds: how can enantiomerism be observed and measured? Fortunately, there is one physical property in which enantiomers differ: their ability to rotate plane-polarized light.
Recall that light consists of a series of vibrating
waves.
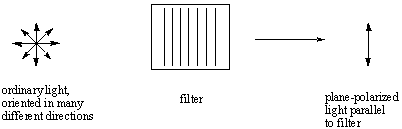
The light that we typically see is unpolarized; that is, it consists of waves
that are oriented in every possible direction in an even distribution. We can
pass unpolarized light through a polarizing filter to obtain plane-polarized
light, which consists of light waves oriented in only a single direction.
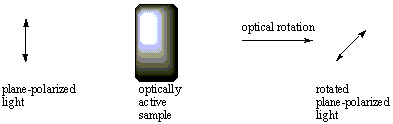
Solutions of chiral compounds have the property of rotating plane-polarized
light passed through them. That is, the angle of the light plane is tilted to
the right or to the left after emerging from the sample. Achiral compounds do
not have this property. The ability of a solution to rotate plane-polarized
light in this fashion is called optical activity, and solutions which have
this ability are said to be optically active.
Using a technique called polarimetry, optical activity is measured by a device called a polarimeter. Monochromatic light (light containing a single color) is filtered through a polarizer to produce plane-polarized light, and it is passed through the sample. A second filter is placed with its slits parallel to those of the first filter, then the sample is rotated until light is transmitted through the second filter. The number of degrees the sample is rotated is called the optical rotation of the sample. If rotation occurs to the right (clockwise), the optical rotation is given a + sign and the sample is considered dextrorotary. If rotation occurs to the left (counter-clockwise), the optical rotation is assigned a--sign and the sample is levorotary.
The optical rotation of a given sample varies with its concentration and the
light's path length: