Problem :
You have 4 moles of O2 and 2 moles of FeS2 and
are given the following balanced equation:
Which reactant is the limiting reagent?
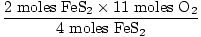 = 5.5 moles O2 = 5.5 moles O2 |
|
5.5 moles O2 are needed to completely react with 2 moles
FeS2. Since we don't have this much
O2,
O2 is the limiting reagent.