Exothermic and Endothermic Reactions
Some stoichiometric calculations involve the change in energy that
accompanies a chemical reaction. Reactions that release energy in the
form of heat are called exothermic reactions. Conversely,
reactions requiring heat energy are known as endothermic reactions.
Here are some examples of exothermic and endothermic reactions:
Exothermic:
| | | C(s) + O2(g)→CO2(g) + 393.5 kJ |
|
| | | SO3(g) + H2O(l)→H2SO4(aq) + 129.6 kJ |
|
Endothermic:
| | | CaCO3(s) + 176 kJ→CaO(s) + CO2(g) |
|
| | | 2CO2(g) + 43.9 kJ→2CO(g) + O2(g) |
|
Reactions like this, which include the amount of heat energy produced
or absorbed, are known as thermochemical equations. Although not
common, note that these equations can also be written as follows:
Exothermic:
| | | C(s) + O2(g) - 393.5 kJ→CO2(g) |
|
| | | SO3(g) + H2O(l) - 129.6 kJ→H2SO4(aq) |
|
Endothermic:
| CaCO3(s) | → | CaO(s) + CO2(g) - 176 kJ2CO2(g) |
|
| | → | 2CO(g) + O2(g) - 43.9 kJ |
|
Heat Energy and Mole Ratios
When calculating mole
ratios
for reactions with given heat energies, you must treat the heat
energy just as you would any other reactant/product in the reaction.
How? Just imagine there is a coefficient of 1 in front of the heat
energy. This amount of heat energy is absorbed/produced in ratio with
the rest of the equation. This concept is illustrated in the following
example problem.
Problem: Based on the following balanced equation, how many kcal
of energy are needed to decompose 200 grams of CaCO3(s)?
| CaCO3(s) + 176 kJ→CaO(s) + CO2(g) |
|
Solution: Convert to moles.
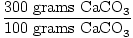 ×1 mole CaCO3 = 3 moles CaCO3 ×1 mole CaCO3 = 3 moles CaCO3 |
|
Now do the mole ratio; 176 kJ are needed for every mole of
CaCO3.
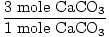 ×176 kJ = 528 kJ ×176 kJ = 528 kJ |
|
Finally, you must put the answer in terms of kcal. There are 4.18 kJ
in every kcal. Use the necessary conversion factor.
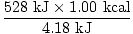 = 126 kcal = 126 kcal |
|