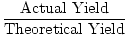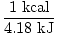-
Actual yield
The amount of product that is actually produced in a chemical reaction.
-
Endothermic reaction
A reaction that requires heat energy to be carried to completion.
-
Enthalpy
The amount of heat a substance has at a given temperature and pressure.
-
Excess reagent
The reactant that is in excess in a chemical reaction. There is more than enough of it to react with the other reactant(s).
-
Exothermic reaction
A reaction that releases energy in the form of heat.
-
Heat of reaction
The change in enthalpy in a chemical reaction.
-
Limiting reagent
The reactant that limits or determines the amount of product that can be formed in a chemical reaction.
-
Percent yield
The decimal percentage resulting from dividing actual yield by theoretical yield.
-
Standard heat of formation
Unique to every substance, it is a measure of the change in enthalpy in the reaction that produces 1 mole of the substance from its constituent elements.
-
Theoretical yield
The calculated or expected amount of product to be formed in a chemical reaction.
-
Thermochemical reaction
Chemical reactions that include the amount of heat energy produced or absorbed.
Formulas
| Formula for Percent Yield |
|
| δH |
|
| Conversion Factor between kJ and kcal |
|