Alternate Definitions of Variables
We originally defined our variables in terms of U, but now that we
have more expressions for the energy, we can devise new expressions of
the variables as well.
For example, we originally defined the
temperature as τ = 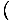
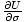
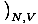 . But we can also use the enthalpy identity to
write τ =
. But we can also use the enthalpy identity to
write τ = 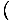
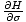
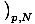 .
.
It isn't hard to formulate these definitions on your own. Find an
energy identity in which the variable you wish to define is free, and
then take the other two differentials constant and solve. Say we wish
to look at the entropy in terms of the
Helmholtz free energy. We see that V and N are in differentials
in F, and so we write: σ = - 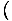
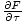
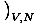 .
.
Many other relations exist, but we will leave you to derive them on your
own and in the problems at the end of the section. Again, understanding
this fluidity and flexibility in definition will be key to solving
problems efficiently.
Alternate Definitions Using the Energy
Above we showed how to define the variables in terms of the energy, but
we can bypass the energy by keeping it constant. For example, suppose
the energy U were held constant, as were the number of particles.
Though we will gloss over some mathematics here, it seems plausible that
you could then write: p = τ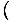
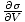
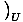 .
.
Moreover, we can use the definitions of the other energies to obtain
more complex formulations of the variables. Take F = U - τσ. We
know that p = - 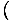
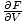
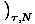 . We can
take the derivative to obtain:
. We can
take the derivative to obtain: