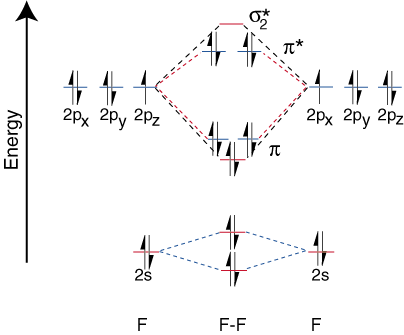
Heteronuclear Diatomic Molecules
To draw the correlation diagrams for heteronuclear diatomic molecules, we face a new problem: where do we place the atomic orbitals on an atom relative to atomic orbitals on other atoms? For example, how can we predict whether a fluorine 2s or a lithium 2s orbital is lower in energy? The answer comes from our understanding of electronegativity. Fluorine is more electronegative than lithium. Then electrons are more stable, i.e. lower in energy, when they are lone pairs on fluorine rather than on lithium. The more electronegative element's orbitals are placed lower on the correlation diagram than those of the more electropositive element. illustrates this point:

Since lithium only has one occupied valence orbital, only one bonding and one antibonding orbital are possible. Furthermore, the electrons in orbitals on F that cannot bond with Li are left on F as lone pairs. As you can see, the electrons in the Li-F s bond are quite close in energy to fluorine's 2p orbitals. Then the bonding orbital is primarily composed of a fluorine 2p orbital, so the M.O. diagram predicts that the bond should be polarized toward fluorine--exactly what is found by measuring the bond dipole. Such an extreme polarization of electron density towards fluorine represents a transfer of an electron from lithium to fluorine and the creation of an ionic compound.
The construction of other heteronuclear diatomic orbital correlation diagrams follows exactly the same principles as those we employed for LiF. To see more examples of such diagrams, consult your favorite chemistry textbook.
Bonding in Polyatomic Molecules
As you can imagine, to describe the bonding in polyatomic molecules, we would need a molecular orbital diagram with more than two dimensions so we could describe the bonds both between the central atom and each terminal atom and between the terminal atoms themselves. Such diagrams are impractically difficult to draw or require complex methods to collapse such multidimensional figures into two dimensions. Instead we will describe a simple yet powerful method to describe the bonding in polyatomic molecules called hybridization. By adding together certain atomic orbitals, we can produce a set of hybridized atomic orbitals that have the correct shape and directionality to account for the known bond angles in polyatomic molecules. Hybrid orbitals describe the bonding in polyatomic molecules one bond at a time.
From the geometry of the molecules, as predicted by VSEPR, we can deduce the hybridization of the central atom. Linear molecules are sp hybridized. Each hybrid orbital is composed of a combination of an s and a p orbital on the central atom. The other geometries are produced by the proper mixture of atomic orbitals. Molecules based on a triangle are sp2 hybridized. Tetrahedrally based molecules are sp3 hybridized. Trigonal bipyramidally based molecules are dsp3 hybridized. Octahedrally based molecules are d2sp3 hybridized.
To illustrate how hybrid orbitals are used to describe the bonding in polyatomic molecules, we will examine the bonds that form water, H2O. Water is AB2e2, therefore, its geometry is based on a tetrahedron, and it is sp3 hybridized. Two sp3 hybrid orbitals on oxygen with one electron each can form a bond with the singly occupied 1s orbitals on the hydrogen atoms. The remaining two sp3 hybrid orbitals on oxygen each have two electrons in them and are, therefore, lone pairs. A model of the bonding in water is shown in :
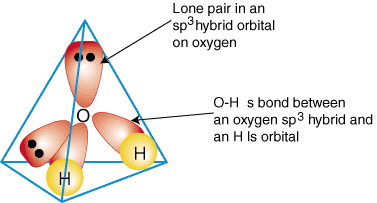
To produce hybrid bonding descriptions of any compound, first decide what is the hybridization of the central atom based on its geometry. Next, form bonds between the hybrid or atomic orbitals on terminal atoms and the central atom. Finally, check to make sure that your bonding description agrees with the Lewis structure in the number of bonds formed and the number of lone pairs.


 payment page
payment page



