Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Properties of Chemical Bonds
Why should atoms bond at all? In nature we find that some elements like He, Ne, and Ar are never found bonded to other atoms whereas most other elements are only found bonded to other elements. What makes the noble gases so special? The answer lies in their closed shell electron configurations. Because the valence shell of a noble gas is completely full, it cannot accept another electron into the shell. The nucleus is positively charged and pulls on the electron, so the loss of an electron from a noble gas is unfavorable. Therefore, like real nobility, the noble gases do not want to do anything at all--that is, noble gases are unreactive because they have filled valence shells.
Any element other than a noble gas has an open shell configuration, which is unstable relative to the configuration of a noble gas. Non-noble atoms react to form bonds in an attempt to achieve a closed shell electron configuration. For example, when a lithium atom and a fluorine atom meet, as shown in , lithium can achieve a noble gas configuration, 1s2, by donating an electron to fluorine which also achieves the noble gas configuration 1s22s22p6:
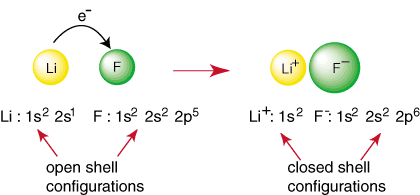
The above reaction represents the formation of an ionic bond. The negatively charged anion, F, and the positively charged cation are held together in the bond by the attraction of unlike charges as dictated by Coulomb's law. You may have asked yourself why two fluorine atoms don't come together to perform the following reaction:
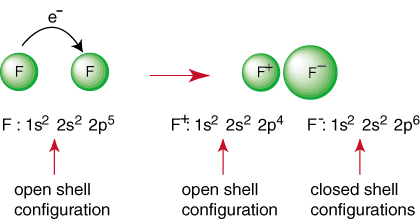
Even though the reaction may appear to be favorable because of its production of a closed shell species, there is a way to have both F atoms achieve a noble gas configuration. By sharing their electrons, each fluorine atoms can have a complete octet in its valence shell. Such a sharing of electrons is called a covalent bond and will be discussed in depth in a separate section.
The way bond properties were chosen to characterize bonds have a historical basis. Scientists made their first rational attempts to describe bonding by looking at data they could collect about bonds. We too will look at the experimental data on bonds to try to analyze bonding.
Perhaps the most useful aspect to know of a bond is its strength. Weak bonds are easily broken and molecules with such bonds are fairly reactive. Conversely, strong bonds are difficult to break and give rise to stable molecules. Therefore, it is sensible to define bond strength as the amount of energy needed to break a chemical bond. Trends in bond strength show that homoatomic bonds (those formed between atoms of the same element) tend to be strong. But going across a row in the periodic table, the trend in bond strength may not be regular. For example, period 2 elements have the following strength order: Li-Li > Be-Be < B-B < C-C < N-N > O-O > F-F. This irregular trend is repeated in period 3 homoatomic bonds. If we look at bond strength data, we also notice that the Li-F bond is several times stronger than the F-F bond or the Li-Li bond. It is not important for you to memorize such trends. We use them to show that whatever theory of covalent bonding we propose must account for these observations.
Please wait while we process your payment





