Gas Density
PV = nRT is an equation, and it can be manipulated just like all other
equations. With this in mind, let's see how the ideal gas law can help us
calculate gas density.
Density d has the units of mass over volume. The ideal gas law transforms
into a form with units in mol per unit volume:
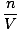 = = 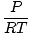 |
|
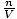
generally has the units of mol per liter. If we multiply both
sides of the equation by the molar mass of the gas,
μ,
we get:
d = 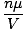 = = 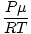 |
|
As we can see from this equation, the density
d of a gas depends
on
P,
μ, and
T. Think about how density will change when the
temperature and pressure rise.
Partial Pressure and Mole Fraction
Dalton's law states that the total pressure of a mixture of gases is the sum
of the pressures each constituent gas would exert if it were alone. Dalton's
law can be expressed mathematically:
| Ptot = PA + PB + PC + ... |
|
Each individual pressure
PA,
PB,
PC, etc. is the pressure exerted by
each constituent gas A, B, or C.
PA is called the partial pressure of
gas A.
Each individual gas obeys the ideal gas law, so we can rearrange PV = nRT
to find pressure:
PA = na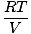 |
|
Since gases A, B, and C are all in the same mixture, they all have the same
temperature and volume.
Ptot also has the same temperature and
volume. When
PA is placed over
Ptot, the variables
T,
R,
and
V cancel to give the following result:
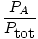 = = 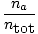 |
|
The quantity
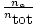
is called the mole fraction of gas
A and is abbreviated
ρA.
Dalton's law problems often present two containers of gas, mix them, and ask you
to find the partial pressures of each gas. There's usually an easy way and a
hard way to do such problems; the trick is finding the easy way. You'll gain
this intuition quickest if you jump right in. Try your hand at the problems at
the end of this section and in your textbook.