Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Conformational Analysis of Cycloalkanes
The key to understanding trends in ring strain is that the atoms in a ring do not necessarily lie flat in a plane. We begin by studying the most stable conformation of cyclohexane, which has completely staggered dihedral angles at each of the six C-C bonds. This conformation is not flat but is folded into the shape of a lawn chair, so it is called the chair conformation.
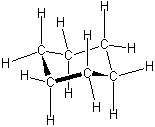
While the chair is typically drawn from such a perspective view, keep in mind that the chair actually has three-fold rotational symmetry. That is, it can be rotated by 120 or 240 degrees and look identical. The best way to visualize the chair conformation is to build one for yourself using a molecular model kit. You should verify that the chair has no eclipsing interactions. This can also be seen in a Newman projection down a set of two C-C bonds. Notice how this resembles two Newman projections of ethane joined together by -CH2- groups:
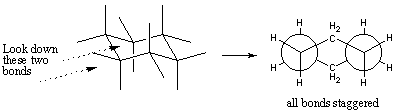
There are two distinctive types of C-H bonds on the chair. One set is comprised of C-H bonds that extend vertically up and down and are called axial bonds. The other set consists of C-H bonds that extend out to the periphery of the ring and are called equatorial bonds. Each carbon has one axial bond and one equatorial bond.
Recall from our discussion of cis-trans isomerism that each carbon has a bond that points up to the top face of the ring and a bond that points down to the bottom face. Don't get these confused with the axial/equatorial classification. One equatorial bond isn't necessarily cis to another equatorial bond, and the same applies for axial bonds. The up/down orientation of an axial position changes from one carbon to its neighbors. If axial is up on one carbon, axial will be down on its two neighbors. The same is true for equatorial positions.

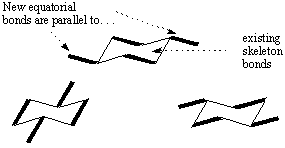
The chair conformation is used so frequently that you should become comfortable
with drawing them. The most important feature of chair conformations is that
they consist of three sets of parallel C-C bonds. Begin by drawing the six C-C
bonds that comprise the skeleton of the chair:
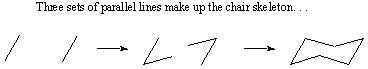
Like other conformations we have studied, chair conformations are in a state of constant
flux. Because all the C-C bonds are interconnected,
they cannot rotate independently but have to move together. For example, one
end of the chair could "flip up" to put the cyclohexane ring in a boat
conformation.
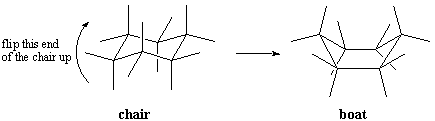
Please wait while we process your payment





