Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews July 8, 2025 July 1, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Conformational Analysis of Cycloalkanes
The boat conformation is less stable than the chair conformation because it
experiences a number of eclipsing interactions. Whereas the chair conformation
resembles two staggered ethanes, the boat conformation resembles two eclipsed
ethanes. In addition, there is considerable repulsion between hydrogens on the
two "tips" of the boat. These hydrogens are called flagpole hydrogens. The
combined effects of torsional strain and steric hindrance between flagpole
hydrogens makes the boat conformation less stable than the chair by 6.9
kcal/mol.
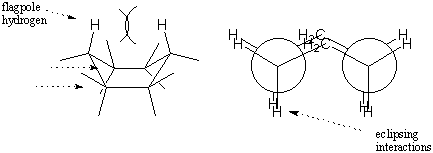
We could flip either end of the boat down to regain a chair conformation. The
two possible chair conformations that can be obtained are distinct; all of the
axial bonds in one chair become equatorial in the other and vice versa. These
two chair conformations can be interconverted by going through the boat
intermediate. Such a chair-chair interconversion is sometimes called a chair
flip. Build a model of cyclohexane with distinct colors for the axial and
equatorial hydrogens. Try the chair flip yourself to verify that the colors
really do change positions. The effect is really quite startling
the first time you see it!
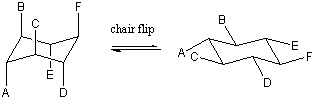
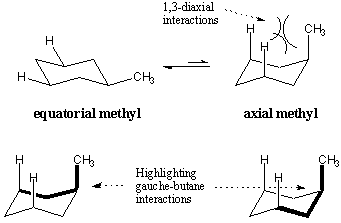
When substituents are placed on the cyclohexane ring, they prefer to take
equatorial positions over axial positions. This positional preference is shown
for methylcyclohexane. When the methyl group occupies the axial position, there
is steric hindrance between it and the axial hydrogens three carbons away.
These repulsive effects are called 1,3-diaxial interactions. 1,3-diaxial
interactions can also be understood in terms of gauche butane. The highlighted
bonds indicate the butane-like structures in the axial conformation of
methylcyclohexane. It turns out that the axial methyl conformation is less
stable by 1.8 kcal/mol, precisely the cost of two gauche butane interactions.
The amount of energy it "costs" to move a substituent group into the axial position is sometimes referred to as the A-value of that substituent group. For instance, the A-value of a methyl group is 1.8 kcal/mol. The A-values of several substituent groups are listed below. A-values can be useful for estimating the energy difference between the two conformations of a substituted cyclohexane. However, a simple summation of A-values does not always give the right answer, as Problem 9 will show.
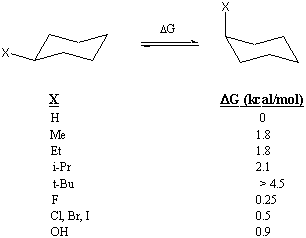
It is useful to know the energy difference between the two chair conformations because it enables you to calculate the relative abundance of each conformation. For instance, the 1.8 kcal/mol A-value of methyl allows us to predict that less than 1 in 20 molecules of methylcyclohexane will occupy the axial position at room temperature. The A-value of the tert-butyl group is so large that any molecule with a tert-butyl substituent is "locked" into the conformation that places the tert-butyl group in the equatorial position.
Please wait while we process your payment

