Lipids and Phospholipids
The structure of the lipid bilayer explains its function as a barrier. Lipids are fats, like oil, that are insoluble in water. There are two important regions of a lipid that provide the structure of the lipid bilayer. Each lipid molecule contains a hydrophilic region, also called a polar head region, and a hydrophobic, or nonpolar tail region.
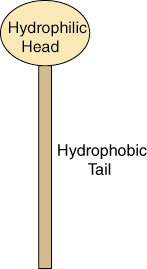
Figure 2.10: Basic Lipid Structure
The hydrophilic region is attracted to aqueous water conditions while the hydrophobic region is repelled from such conditions. Since a lipid molecule contains regions that are both polar and nonpolar, they are called amphipathic molecules.
The most abundant class of lipid molecule found in cell membranes is the phospholipid. The phospholipid molecule's polar head group contains a phosphate group. It also sports two nonpolar fatty acid chain groups as its tail.

Figure 2.11: Phospholipid Structure
The fatty acid tail is composed of a string of carbons and hydrogens. It has a kink in one of the chains because of its double-bond structure.
The Bilayer
The phospholipids organize themselves in a bilayer to hide their hydrophobic tail regions and expose the hydrophilic regions to water. This organization is spontaneous, meaning it is a natural process and does not require energy. This structure forms the layer that is the wall between the inside and outside of the cell.

Figure 2.12: Lipid Bilayer
Properties of the Lipid Bilayer
As we have already mentioned, the most important property of the lipid bilayer is that it is a semi-permeable structure. Semi-permeable simply means that it only allows some molecules to freely pass across it. This property means that large molecules, like proteins, polar molecules, ions, and charged molecules cannot cross the bilayer, and thus the cell membrane, without the assistance of other structures. However, water and gases can easily pass through the bilayer. These gases, such as N2, CO2, and O2, are able to pass through the membrane because they are non-polar like the non-polar fatty acid tails. Water is able to pass through the membrane in small amounts because it is a small, polar molecule. This allows it to “sneak” bast the hydrophilic tails, taking advantage of the fluidity of the membrane.
Another important property of the lipid bilayer is its fluidity. The lipid bilayer contains lipid molecules, and, as we will discuss later, it also contains proteins. The bilayer's fluidity allows these structures mobility within the lipid bilayer. This fluidity is biologically important, influencing membrane transport.
Structurally, the lipid bilayer is asymmetrical: the lipid and protein composition in each of the two layers is different.













