Most reactions have an activation energy associated with them. As represented in Figure 3.2, activation energy is the amount of energy needed for a chemical reaction to occur. Enzymes act as biological catalysts, facilitating chemical reactions in cells by lowering the activation energy. Lowering this activation energy allows reactions to occur faster and at lower temperatures, as too much heat would result in the death of the cell. Enzymes also create a mechanism for regulating biological processes.
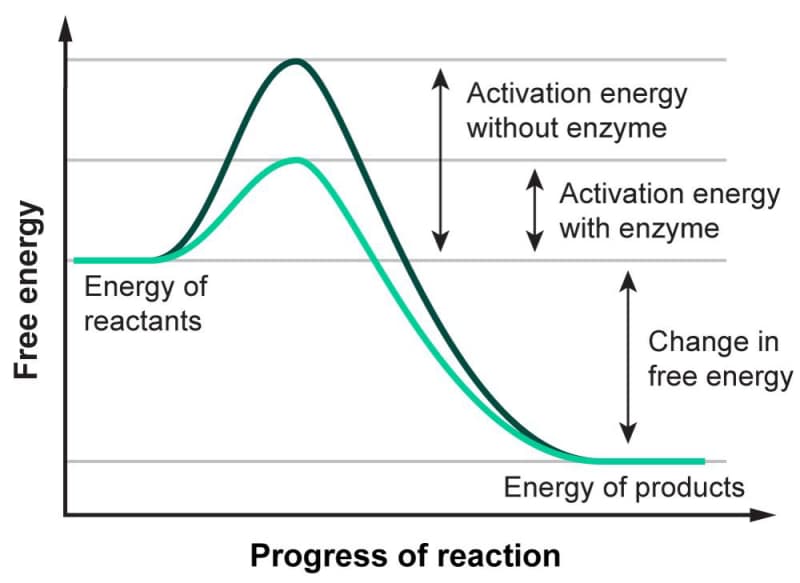
Figure 3.02: Activation Energy Graph showing the difference in energy required with and without an enzyme. The x-axis shows the progress of the reaction, and the y-axis represents the energy of the molecules involved in the reaction. As you can see, there is an initial energy that needs to be supplied for the reaction to move forward. This amount of energy is much lower with an enzyme present.
Catalysis
During enzyme catalysis, substrate(s) bind to an enzyme’s active site to form the enzyme-substrate complex. During the reaction, the enzyme helps stabilize the transition state which makes it easier for the reaction to proceed. When the reaction is completed, the product leaves the enzyme, and the enzyme is now available for another substrate to bind. Enzymes are not affected by the reactions they catalyze and so can be reused.













