Structure of Water
Water molecules consist of one oxygen atom and two hydrogen atoms (Figure 1.1). Due to this structure, there is unequal sharing of electrons which creates a partial negative charge on the oxygen and partial positive charges on the hydrogen atoms. This polarity makes water an excellent solvent because it can interact with charged and polar substances. This allows water to dissolve substances and be the solvent that life exists within. The polarity of water also allows for hydrogen bonding between water molecules. Hydrogen bonds are interactions between the positively charged hydrogen atom of one water molecule and the negatively charged oxygen atom of another water molecule. While water is also unique in its thermodynamic properties and ability to transition easily between solid, liquid, and gas, we will focus on the liquid properties of water as that is the state that occurs in living organisms.
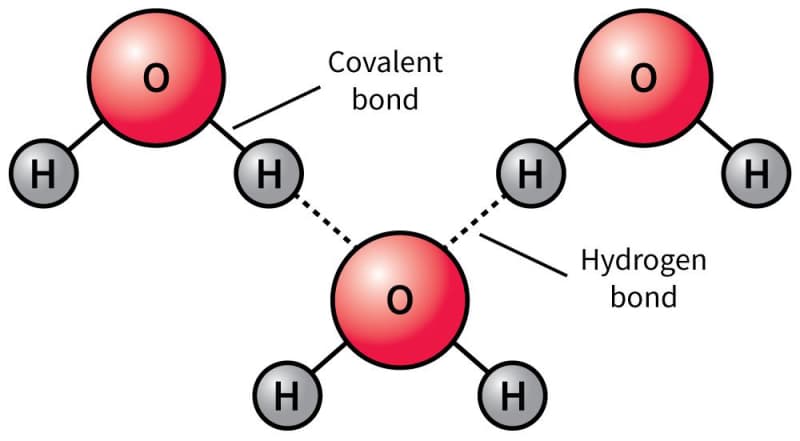
Figure 1.01: The structure of a water molecule and the bonds they form with other water molecules.
Properties of Water
Living systems depend on properties of water that result from its polarity and hydrogen bonding. Hydrogen bonds are weak individually, but together their effects are significant. The hydrogen bonds between water molecules result in three main properties: cohesion, adhesion, and surface tension. Cohesion is the attraction of water molecules to each other. This causes water molecules to stick together. An example of this is surface tension. Surface tension is the tendency of water, when in its liquid state, to shrink into the minimum surface area possible. This property of water is what creates an “invisible layer” on the surface of water and allows some small organisms like water striders to walk on the surface. It is also what allows a glass of water to be filled above the brim and yet not overflow. Adhesion is the attraction of water molecules to other substances. This allows water to interact with other surfaces. Both of these properties are critical in maintaining the integrity of water and moving molecules around within cells and organisms. Water also supports and participates in key biochemical reactions that occur in cells. For example, water molecules are often used to break bonds in larger molecules (hydrolysis) or water molecules are produced when smaller molecules are connected (dehydration synthesis).













