Nucleotides and Nucleic Acids
Both DNA and RNA are known as nucleic acids. They have been given this name for the simple reason that they are made up of structures called nucleotides. Those nucleotides, themselves comprising a number of components, bond together to form the double-helix first discovered in 1953 by the scientists James Watson and Francis Crick with significant contributions from fellow researchers Rosalind Frankin and Maurice Wilkins.
A nucleotide consists of three things:
-
A nitrogenous base, which can be either adenine, guanine, cytosine, or thymine. In the case of RNA, thymine is replaced by uracil.
-
A five-carbon sugar, deoxyribose in DNA and ribose in RNA. Deoxyribose is named such because it is lacking an oxygen group on one of its carbons.
-
One phosphate group.
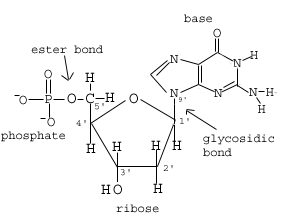

Figure 1.02: A nucleotide
The nitrogen bases and form a glycosidic bond with the deoxyribose. This type of bond is called a glycosidic bond. The phosphate group forms an ester bond with the deoxyribose sugar (Figure 1.02).
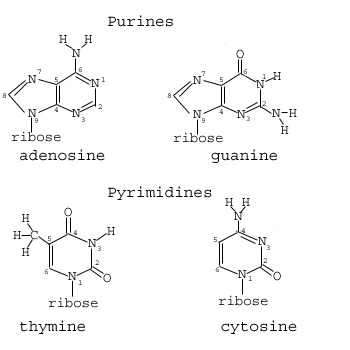
Figure 1.03: DNA Bases
As mentioned above, there are five kinds of nucleotides. Each of these bases are often abbreviated a single letter: A (adenine), C (cytosine), G (guanine), T (thymine). The bases come in two categories: thymine and cytosine are pyrimidines, while adenine and guanine are purines (Figure 1.03). The pyrimidine structure is produced by a six-membered, two-nitrogen molecule; purine refers to a nine-membered, four-nitrogen molecule. As you can see, each constituent of the ring making up the base is numbered to help with specificity of identification.
The uracil base replaces thymine in RNA. Thymine and uracil are structurally very similar. Uracil has fundamentally the same structure as thymine, with the deletion of a methyl group at the 5' position.
Nucleotides join together through phosphodiester linkages to form nucleic acids. The hydroxyl group of the sugar forms the bond with one of the oxygens of a phosphate group in a different nucleotide. When many of these nucleotide subunits combine, the result is the large single-stranded polynucleotide or nucleic acid (Figure 1.04).

Figure 1.04: Example of a nucleic acid chain
If you look closely, you can see that the two ends of the nucleic acid strand shown above are different, resulting in directionality. At one end of the molecule, the 5’ carbon is unbound and at the other end the 3’ hydroxyl group is unbound. These different ends are called the 5'- and 3'-ends, respectively.
Structure of DNA
Figure 1.04 shows a single strand of DNA. However, DNA usually exists as a double-helix, meaning two strands of DNA bind together (Figure 1.05). This is different than RNA that is typically single stranded.

Figure 1.05: Double-helical DNA
When in the double-stranded form, one strand is oriented in the 5' to 3' direction while the complementary strand runs in the 3' to 5' direction. Because the two strands are oppositely oriented, they are said to be antiparallel to each other. The two strands bond through their nitrogen bases (marked A, C, G, or T for adenine, cytosine, guanine, and thymine). Note that adenine only bonds with thymine, and cytosine only bonds with guanine. Chargaff's rule states that the ratio of A to T and of G to C is equal in a DNA molecule. Chargaff's Rule is true as a result of the strict hydrogen bond forming rules in base pairing. For every G in a double-strand of DNA, there must be an accompanying complementary C, similarly, for each A, there is a complementary paired T.
The nitrogen bases are held together by hydrogen bonds: adenine and thymine form two hydrogen bonds; cytosine and guanine form three hydrogen bonds. Hydrogen bonding is essential to the three-dimensional structure of DNA. These bonds do not, however, contribute largely to the stability of the double helix. Hydrogen bonds are very weak interactions and the orientation of the bases must be just right for the interactions to take place. While the large number of hydrogen bonds present in a double helix of DNA leads to a cumulative effect of stability, it is the interactions gained through the stacking of the base pairs that leads to most of the helical stability.
Hydrogen bonding is most important for the specificity of the helix. Since the hydrogen bonds rely on strict patterns of hydrogen bond donors and acceptors, and because these structures must be in just the right spots, hydrogen bonding allows for only complementary strands to come together: A- T, and C-G. This complementary nature allows DNA to carry the information that it does.
Structure of RNA
Structurally, DNA and RNA are nearly identical. As mentioned earlier, however, there are three fundamental differences that account for the very different functions of the two molecules.
-
RNA is typically a single-stranded nucleic acid.
-
RNA has a ribose sugar instead of a deoxyribose sugar like DNA.
-
RNA nucleotides have the uracil base instead of thymine. Uracil will base pair with adenine in the same way as thymine pairs with adenine.
Other than these differences, DNA and RNA are very similar. Their phosphates, sugars, and bases show the same bonding patterns to form nucleotides and their nucleotides bind to form nucleic acids in the same way.

Figure 1.06: Structure of RNA
Function
Nucleic acids serve a variety of functions within a cell. DNA is contained within the nucleus and stores information for the growth, functioning, and reproduction of organisms. It is the instruction manual for how to create the proteins needed for life. DNA is also the genetic material that is passed down to offspring. RNA is more of an intermediary molecule. DNA is translated into RNA which then converts the information into proteins. RNA acts as a messenger and copies the information stored in DNA, moves it out of the nucleus, and instructs the cell how to order the amino acids in a protein. There are multiple kinds of RNA which all play a slightly different role in the cell. These will be discussed in more detail in Unit 6 – Gene Expression and Regulation.
Now that we have reviewed the main kinds of macromolecules in a cell, the next unit will go into more detail about the components of cells and how they function.













