Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Atoms and Atomic Orbitals
The spin quantum number tells whether a given electron is spin up (+1/2) or spin down (-1/2). An orbital contains two electrons, and each of those electrons must have different spins.
It is often convenient to depict orbitals in an orbital energy diagram, as seen below in . Such diagrams show the orbitals and their electron occupancies, as well as any orbital interactions that exist. In this case we have the orbitals of the hydrogen atom with electrons omitted. The first electron shell (n = 1) contains just the 1s orbital. The second shell (n = 2) holds a 2s orbital and three 2p orbitals. The third shell (n = 3) holds one 3s orbital, three 3p orbitals, and five 3d orbitals, and so forth. Note that the relative spacing between orbitals becomes smaller for larger n. In fact, as n gets large the spacing becomes infinitesimally small.
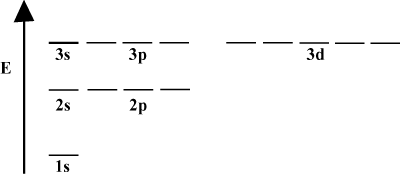
You will see such energy diagrams quite often in your continuing study of chemistry. Notice that all orbitals with the same n have the same energy. Orbitals with identical energies are said to be degenerate (not in the moral sense!). Electrons in higher-level orbitals have more potential energy and are more reactive, i.e. more likely to undergo chemical reactions.
When an atom only contains a single electron, its orbital energies depend only on the principle quantum numbers: a 2s orbital would be degenerate with a 2p orbital. However, this degeneracy is broken when an atom has more than one electron. This is due to the fact that the attractive nuclear force any electron feels is shielded by the other electrons. s-orbitals tend to be closer to the nucleus than p-orbitals and don't get as much shielding, and hence become lower in energy. This process of breaking degeneracies within a shell is known as splitting. In general s orbitals are lowest in energy, followed by p orbitals, d orbitals, and so forth.
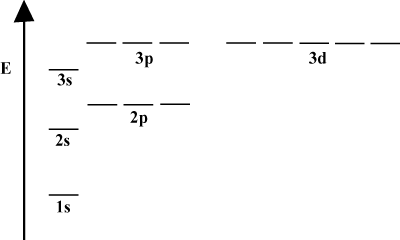
The energy diagram of imply a further fact about the energy of electrons. Note that the energy levels in these diagrams do not follow a continuous line: an atom is either in one energy subshell or it is in another. There is no in between. In this way, the diagram perfectly represents the quantized nature of electrons, meaning that electrons can only exist at specific and defined energy levels. The energy level of an electron in a particular energy shell can be determined according to the following equation:
| En = /frac-2.178x10-18joulesn2 |
Please wait while we process your payment





