Gases
The first step to understanding gases is to spell out what exactly a gas is. Gases have two properties that set them apart from solids and liquids. First, gases spontaneously expand to fill the container they occupy, no matter its size. In other words, a gas has no fixed volume or shape. Secondly, gases are easily compressible.
You can imagine a gas as a busy swarm of molecules. Each molecule moves randomly and travels great distances before bouncing off another molecule. This occurs because the individual molecules comprising a gas are generally far apart. In fact, for a gas at low pressure, we can approximate that aside from a few random collisions, individual gas molecules do not interact. This approximation is what separates gases from solids and liquids, whose molecules always interact. The series of SparkNotes on Gases SparkNote seek to use this approximation about gases to establish the ideal gas law and the kinetic molecular theory. The ideal gas law macroscopically describes how gases behave under nearly all conditions. The kinetic molecular theory describes how sub-microscopic gas molecules interact with each other.
Pressure
Of the three general terms used to describe gases (volume, temperature, pressure), pressure is the least familiar. Before we can delve into the gas theories, we need a firm understanding of it. Pressure is defined as force divided by the area over which the force acts:
pressure
P = 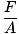 |
Ice skates are familiar examples of the effects of pressure. The area of the blades of a skate are much smaller than, say, the soles of your feet. So if you strap on ice skates, your weight will act on an area much smaller than it would if you were wearing normal shoes. Since A decreases while F stays the same, by @@Equation@@, the pressure you exert on the ice will be much greater if you're wearing skates. This pressure is often enough to melt a layer of ice, which allows your skate to glide smoothly across an ice rink. If you try the same maneuver with normal shoes, you will not generate enough pressure to melt the ice and won't get anywhere fast.
So how does pressure relate to gases? If you will remember, a gas will fill any container that holds it. It is easy to see why with our swarm analogy. If a compact swarm of molecules is placed into a large container, the individual molecules will move about randomly and eventually stray from their original dimensions. Eventually, some intrepid molecules will reach the walls of the container. When they do, they will impact the walls of the container. These impacts generate a force, and hence a pressure on the walls of the container.













