-
van der Waals equation
The van der Waals equation corrects the ideal gas law for real behavior:
(P + 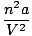 )(V - nb) = nRT
)(V - nb) = nRT
-
Intermolecular
An event that occurs between two discrete molecules.
Terms
The van der Waals equation corrects the ideal gas law for real behavior:
(P + 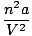 )(V - nb) = nRT )(V - nb) = nRT |
An event that occurs between two discrete molecules.