Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Conformational Analysis of Cycloalkanes
The boat conformation is less stable than the chair conformation because it
experiences a number of eclipsing interactions. Whereas the chair conformation
resembles two staggered ethanes, the boat conformation resembles two eclipsed
ethanes. In addition, there is considerable repulsion between hydrogens on the
two "tips" of the boat. These hydrogens are called flagpole hydrogens. The
combined effects of torsional strain and steric hindrance between flagpole
hydrogens makes the boat conformation less stable than the chair by 6.9
kcal/mol.
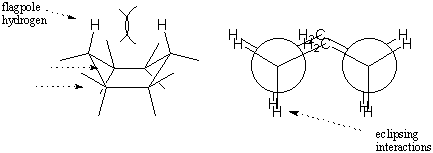
We could flip either end of the boat down to regain a chair conformation. The
two possible chair conformations that can be obtained are distinct; all of the
axial bonds in one chair become equatorial in the other and vice versa. These
two chair conformations can be interconverted by going through the boat
intermediate. Such a chair-chair interconversion is sometimes called a chair
flip. Build a model of cyclohexane with distinct colors for the axial and
equatorial hydrogens. Try the chair flip yourself to verify that the colors
really do change positions. The effect is really quite startling
the first time you see it!
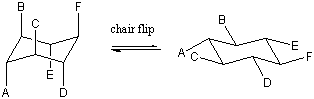
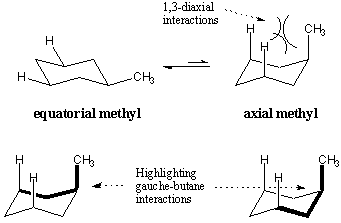
When substituents are placed on the cyclohexane ring, they prefer to take
equatorial positions over axial positions. This positional preference is shown
for methylcyclohexane. When the methyl group occupies the axial position, there
is steric hindrance between it and the axial hydrogens three carbons away.
These repulsive effects are called 1,3-diaxial interactions. 1,3-diaxial
interactions can also be understood in terms of gauche butane. The highlighted
bonds indicate the butane-like structures in the axial conformation of
methylcyclohexane. It turns out that the axial methyl conformation is less
stable by 1.8 kcal/mol, precisely the cost of two gauche butane interactions.
The amount of energy it "costs" to move a substituent group into the axial position is sometimes referred to as the A-value of that substituent group. For instance, the A-value of a methyl group is 1.8 kcal/mol. The A-values of several substituent groups are listed below. A-values can be useful for estimating the energy difference between the two conformations of a substituted cyclohexane. However, a simple summation of A-values does not always give the right answer, as Problem 9 will show.
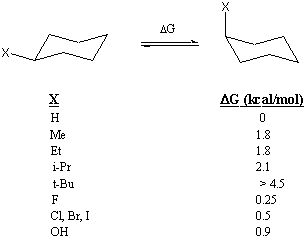
It is useful to know the energy difference between the two chair conformations because it enables you to calculate the relative abundance of each conformation. For instance, the 1.8 kcal/mol A-value of methyl allows us to predict that less than 1 in 20 molecules of methylcyclohexane will occupy the axial position at room temperature. The A-value of the tert-butyl group is so large that any molecule with a tert-butyl substituent is "locked" into the conformation that places the tert-butyl group in the equatorial position.
Please wait while we process your payment





