This result is interesting because we can think of the first term as the
pressure due to energy and the second as the pressure due to the
entropy.
Maxwell Relations
What started as a simple picture may be looking confusing now, but
remember that underneath all of the new equations that are arising is
the Thermodynamic Identity, and the definitions of the other energies
from that. Everything else follows from those and can be rederived
without much difficulty.
We can utilize yet another mathematical truth, that double derivatives
commute, to derive a new set of relations known as the Maxwell Relations.
We know, for example, that 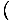
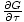
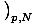 = - σ and
that
= - σ and
that 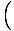
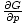
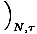 = V, by
the methodology just discussed. Now, though, we can take the partial
derivative of the first equation with respect to p, holding τ
constant, to obtain:
= V, by
the methodology just discussed. Now, though, we can take the partial
derivative of the first equation with respect to p, holding τ
constant, to obtain:
Similarly, we can take the partial derivative of the second equation
with respect to τ, holding p constant, to obtain:
The mathematical truth useful here is that: