Boyle's Law
The most important thing to remember about Boyle's Law is that it only holds when the temperature and amount of gas are constant. A state of constant temperature is often referred to as isothermal conditions. When these two conditions are met, Boyle's law states that the volume V of a gas varies inversely with its pressure P. The equation below expresses Boyle's law mathematically:
| PV = C |
C is a constant unique to the temperature and mass of gas involved. plots pressure versus volume for a gas that obeys Boyles law.
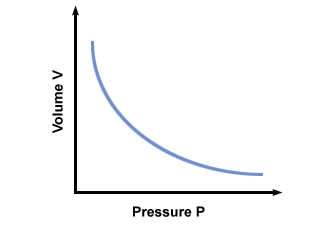
You will get the most mileage out of another incarnation of Boyle's law:
| P1V1 = P2V2 |
The subscripts 1 and 2 refer to two different sets of conditions. It is easiest to think of the above equation as a "before and after" equation. Initially the gas has volume and pressure V1 and P1. After some event, the gas has volume and pressure V2 and P2. Often you will be given three of these variables and asked to find the fourth. You should realize that this is a simple case of algebra. Separate the knowns and unknowns on two different sides of the "=" sign, plug in the known values, and solve for the unknown.
The Manometer
Boyle used a manometer to discover his gas law. His manometer had an odd
"J" shape:
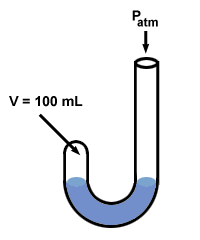
Next Boyle added mercury to the open end of his manometer.
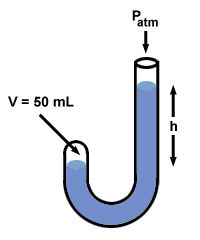
| P1V1 = P2V2 |
The pressure of the gas before mercury is added is equal to the atmospheric pressure, 760 mm Hg (let's assume that the experiment is run at oC so that 1 torr = 1 mm Hg). So P1 = 760 mm Hg. The volume V1 is measured to be 100 mL.
After Boyle added mercury, the volume of the gas, V2, drops to 50 mL. To find the value of P2, rearrange the equation above and plug in values:
| P2 | = | P1V1/V2 | |
| = | (100 mL)(760 mm Hg)/(50 mL) | ||
| = | 1520 mm Hg |
If you look back at , you'll notice that the difference P2 - P1 = 760 mm Hg, and that this exactly equals the difference in mercury levels on the two sides, h. In fact, Boyle's manometer illustrates a truism common to all manometers: h corresponds to the difference in pressure between the two ends of the manometer.
Boyle's manometer is only one of the many kinds of manometers you'll face. Don't be disheartened; all manometers are practically the same. Realize that each end of a manometer can only be:
- sealed and contain a vacuum (P = 0)
- open to the atmosphere (P = Patm)
- open to a sample of gas with pressure P
Let's try this procedure with a manometer in which one end is open to the
atmosphere (760 mm Hg) and the other is sealed off to a vacuum.
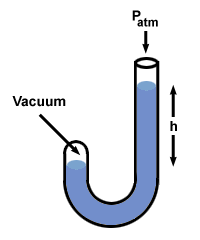
There are a few other flavors of manometer, but you can handle them if you remember that h is the pressure difference between the two sides of the manometer. Note that the side of the manometer with the highest pressure also has the lowest level of Hg.


 payment page
payment page



