-
Conversion Factor
A ratio (or fraction) that represents the relationship between two different units.
-
Formula Unit
The representative particle of a substance. It is the smallest unit of a substance that still retains that substance's properties and is the simplest way to write the formula of the substance without coefficients.
-
Gram Formula Mass
The mass of one mole of compound.
-
Gram Molecular Mass
The mass of one mole of a molecular substance.
-
Mole
A measure of amount. 1 mole equals 6.02×1023 items of something. The number is so massive because moles are used to measure the amount of molecules, atoms, and other incredibly miniscule particles.
-
Mole ratio
The ratio between any two constituents in chemical equation
-
Standard Temperature and Pressure
The arbitrarily decided standard conditions at which experiments are done: 1 atm and 273 degrees Kelvin (0 degrees Celsius).
-
General Conversion Factor Equation
Conversion Factor = 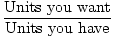
-
Converting from Formula Units to Moles
Moles = 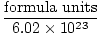
-
Converting from Volume of a Gas to Moles
moles = 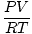
-
Converting from Mass to Moles
Moles = 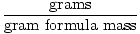
-
Converting from Molality of a Solution to Moles
Moles = molality × kilograms of solution
-
Converting from Molarity of a Solution to Moles
Moles = molarity × liters of solution













