-
Carnot Cycle
A simplified model of the cycle utilized by a heat engine to convert heat into work.
-
Carnot Efficiency
The Carnot efficiency, ηC, is the ratio of the work to the input heat: ηCâÉá
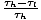
-
Carnot Inequality
The Carnot Inequality states that the actual efficiency of a system is at best equal to the Carnot Efficiency: η≤ηC.
-
Heat
Heat is the transfer of energy to a system via thermal contact with a reservoir.
-
Isentropic
At a constant entropy.
-
Isobaric
At a constant pressure.
-
Isothermal
At a constant temperature.
-
Work
Work is the transfer of energy to a system via a change in the parameters of the system.


 payment page
payment page



