Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

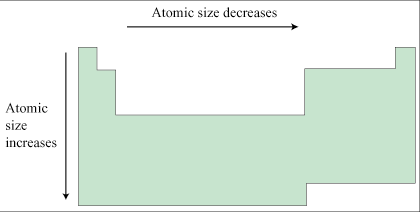
Periodic Trends
Cations and anions do not actually represent a periodic trend in terms of atomic radius, but they do affect atomic radius, and so we will discuss them here.
A cation is positively charged, meaning that it is an atom that has lost an electron or electrons. The positive charge of the nucleus is thus distributed over a smaller number of electrons and electron-electron repulsion is decreased, meaning that the electrons are held more tightly and the atomic radius is smaller than in the normal neutral atom. Anions, conversely, are negatively charged ions: atoms that have gained electrons. In anions, electron-electron repulsion increases and the positive charge of the nucleus is distributed over a large number of electrons. Anions have a greater atomic radius than the neutral atom from which they derive.
The process of gaining or losing an electron requires energy. There are two common ways to measure this energy change: ionization energy and electron affinity.
The ionization energy is the energy it takes to fully remove an electron from the atom. When several electrons are removed from an atom, the energy that it takes to remove the first electron is called the first ionization energy, the energy it takes to remove the second electron is the second ionization energy, and so on. In general, the second ionization energy is greater than first ionization energy. This is because the first electron removed feels the effect of shielding by the second electron and is therefore less strongly attracted to the nucleus. If a particular ionization energy follows a previous electron loss that emptied a subshell, the next ionization energy will take a rather large leap, rather than follow its normal gently increasing trend. This fact helps to show that just as electrons are more stable when they have a full valence shell, they are also relatively more stable when they at least have a full subshell.
Ionization Energy Across a Period
Ionization energy predictably increases moving across the periodic table from left to right. Just as we described in the case of atomic size, moving from left to right, the number of protons increases. The electrons also increase in number, but without adding new shells or shielding. From left to right, the electrons therefore become more tightly held meaning it takes more energy to pry them loose. This fact gives a physical basis to the octet rule, which states that elements with few valence electrons (those on the left of the periodic table) readily give those electrons up in order to attain a full octet within their inner shells, while those with many valence electrons tend to gain electrons. The electrons on the left tend to lose electrons since their ionization energy is so low (it takes such little energy to remove an electron) while those on the right tend to gain electrons since their nucleus has a powerful positive force and their ionization energy is high. Note that ionization energy does show a sensitivity to the filling of subshells; in moving from group 12 to group 13 for example, after the d shell has been filled, ionization energy actually drops. In general, though, the trend is of increasing ionziation energy from left to right.
Please wait while we process your payment





