Ionization Energy Down a Group
Ionization energy decreases moving down a group for the same reason atomic size increases: electrons add new shells creating extra shielding that supersedes the addition of protons. The atomic radius increases, as does the energy of the valence electrons. This means it takes less energy to remove an electron, which is what ionization energy measures.
Electron Affinity
An atom's electron affinity is the energy change in an atom when that atom gains an electron. The sign of the electron affinity can be confusing. When an atom gains an electron and becomes more stable, its potential energy decreases: upon gaining an electron the atom gives off energy and the electron affinity is negative. When an atom becomes less stable upon gaining an electron, its potential energy increases, which implies that the atom gains energy as it acquires the electron. In such a case, the atom's electron affinity is positive. An atom with a negative electron affinity is far more likely to gain electrons.
Electron Affinities Across a Period
Electron affinities becoming increasingly negative from left to right. Just as in ionization energy, this trend conforms to and helps explain the octet rule. The octet rule states that atoms with close to full valence shells will tend to gain electrons. Such atoms are located on the right of the periodic table and have very negative electron affinities, meaning they give off a great deal of energy upon gaining an electron and become more stable. Be careful, though: the nobel gases, located in the extreme right hand column of the periodic table do not conform to this trend. Noble gases have full valence shells, are very stable, and do not want to add more electrons: noble gas electron affinities are positive. Similarly, atoms with full subshells also have more positive electron affinities (are less attractive of electrons) than the elements around them.
Electron Affinities Down a Group
Electron affinities change little moving down a group, though they do generally become slightly more positive (less attractive toward electrons). The biggest exception to this rule are the third period elements, which often have more negative electron affinities than the corresponding elements in the second period. For this reason, Chlorine, Cl, (group VIIa and period 3) has the most negative electron affinity.
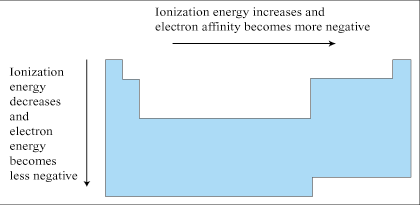
Electronegativity refers to the ability of an atom to attract the electrons of another atom to it when those two atoms are associated through a bond. Electronegativity is based on an atom's ionization energy and electron affinity. For that reason, electronegativity follows similar trends as its two constituent measures.
Electronegativity generally increases moving across a period and decreases moving down a group. Flourine (F), in group VIIa and period 2, is the most powerfully electronegative of the elements. Electronegativity plays a very large role in the processes of Chemical Bonding.


 payment page
payment page



