Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Fundamentals of Rate Laws
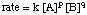
Note that the exponents are not a and b but some experimentally determined powers p and q which may or may not equal a and b. The order of the is, therefore, p + q. We will discuss in Determining Rate Laws how those exponents can be determined.
Also notice in the the presence of the rate constant k. Students often have trouble distinguishing between the rate of a reaction and its rate constant. The rate of a reaction is the total rate of a reaction and is the "rate" in the rate law. The units of rate are always M / s. The rate constant, k, is an experimentally determined proportionality constant that gives some measure of the intrinsic "reactivity" of the reaction. The units on the rate constant depend on the order of the reaction, n, such that k has units M1 - n / s. In that way, the units of the rate constant, k, are chosen to make the units of rate always M / s regardless of order. For example, if the order of the reaction is 3, the rate law could be rate = k [A]2[B]. The units of rate are M / s and the rate constant must, therefore, have units of M-2 / s.
The attentive reader may have noticed that we have only considered the rate of the forward reaction, neglecting any sort of reverse reaction in the rate law. To make our math easier, we have intentionally ignored the reverse reaction and we will continue to do so. This is a justified practice for reactions with negligible reverse rates, such as those with equilibrium constants, K, much greater than 1. For reactions with K's around 1, this is also a valid approximation because they are usually carried out such that products are removed from the reaction mixture as they are formed, keeping concentrations of products low.
Rate laws can also be expressed to relate the concentration of reactants to the time of the reaction. Such an expression is called an integrated rate law because it is the integral of the differential rate law. For those of you who have had some calculus, we present a derivation of an integrated rate law from the differential rate law below for a first order reaction. If you have not had calculus, don't worry. Just pay attention to the end result of the derivation.
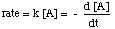
Rearranging this equation gives the following:
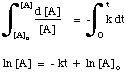
Using a similar technique, one can derive the integrated rate law for any differential rate law. As we will see in Determining Rate Laws, the fact that the 1st order integrated rate law is linear allows us to test if a reaction is first order by plotting ln [A] versus time.
Please wait while we process your payment





