Covalent Bonds
Ionic bonds hold atoms together
through
electrostatic forces. Covalent
bonds operate through an entirely different means: the sharing of electrons.
By sharing electrons, two atoms can mutually complete their valence
shells to become more stable.
A molecule is a collection of atoms held together by
covalent bonds. For example, below, two hydrogen atoms, each with a single
electron, can share their electrons to form a covalent bond and create the
diatomic hydrogen molecule. In this molecular state, both individual hydrogen
atoms attain the noble gas configuration of Helium. Note: the simplest
way to represent molecules is to use a Lewis Dot structure, which is what
you see in the diagram below. We will explain how to draw Lewis
structures later on in this section.
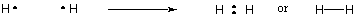
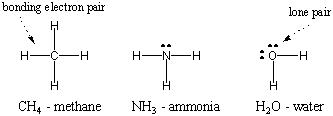
Since hydrogen is the simplest of atoms, having only one electron, diatomic hydrogen is the simplest of molecules, having only a single covalent bond. When more complex atoms form covalent bonds, the molecules they form are also more complex, involving numerous covalent bonds. In some instances, an atom will have valence electrons that are not involved in bonding. These valence electrons are known as lone pairs. The Lewis structures of some common atoms are shown below. Notice that each structure satisfies the octet rule for all its atoms.
Multiple Bonding
When two atoms share a single pair of electrons, the bond is referred to as
a single bond. Atoms can also share two or three pairs of electrons in the
aptly named double and triple bonds. The first bond between two atoms is called
the σ (sigma) bond. All subsequent bonds are referred to as Π (pi)
bonds. In
Lewis structures, multiple bonds are depicted by two or three lines between the
bonded atoms.
The bond order of a covalent interaction between two atoms is the number of
electron pairs that are shared between them. Single bonds have a bond order of
1, double bonds 2, and triple bonds 3. Bond order is directly related to bond
strength and bond length. Higher order bonds are stronger and shorter, while
lower order bonds are weaker and longer.
/PARAGAPH
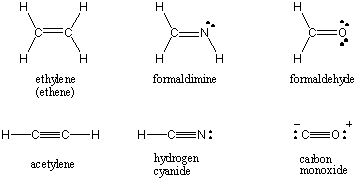
The Lewis structures for some common molecules involving multiple covalent bonds
can be found below.
Electronegativity and Bond Polarity
Not all covalent bonds are fit for Sesame Street: some covalent bonds are shared unequally. Some atoms have a greater ability to attract electrons to themselves than do others. The tendency for an atom to attract electrons is its electronegativity. Don't confuse electronegativity with electron affinity. While both are periodic properties that exhibit similar trends, electron affinity is a measure of energy whereas electronegativity is simply a measure of attraction based on an arbitrary scale. Fluorine, at the upper right hand corner of the periodic table, is the most electronegative element and is assigned an electronegativity of 4.0 while other elements are regarded relative to fluorine. Electronegativity increases from left to right across the periodic table, and decreases as you move down a group.
When covalent bonds are formed between atoms of different electronegativities
the result is that the shared electrons skew more toward one atom than the
other. The resulting molecule is a dipole: the more electronegative atom
in the bond gains a partial negative charge while the less
electronegative atom becomes partially positive. The resulting bond is called a
polar covalent bond. In Lewis structures, a cross-ended arrow is used to
represent such polar bonds, with the arrow pointing to the more electronegative
element. Partial charge symbols δ+ and δ- are used to represent
polarity.
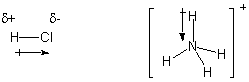
Dipole Moment
Molecules with polar covalent bonds can result in molecules with overall polar attributes. The measure of the overall polarity of a molecule is called the dipole moment. As the value of the dipole moment increases, so does the polarity of the molecule.













