Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



Create Account
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Log into your PLUS account
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
Select Your Plan
Monthly
$5.99
/month + tax
Annual
$29.99
/year + taxAnnual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
Monthly
$5.99
/month + taxYou could save over 50%
by choosing an Annual Plan!

Annual
$29.99
/year + taxSAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
Annual
$22.49/month + tax
Save 25%
on 2-49 accounts
Annual
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
Testimonials from SparkNotes Customers
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Testimonials from SparkNotes Customers
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
Payment Information
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Thank You!
Your group members can use the joining link below to redeem their membership. They will be prompted to log into an existing account or to create a new account. All members under 16 will be required to obtain a parent's consent sent via link in an email.Your Child’s Free Trial Starts Now!
Thank you for completing the sign-up process. Your child’s SparkNotes PLUS login credentials are [email] and the associated password. If you have any questions, please visit our help center.Your Free Trial Starts Now!
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment
Month
Day
Year
Please read our terms and privacy policy
Please wait while we process your payment

Racemic Mixtures and Enantiomeric Excess
A solution in which both enantiomers of a compound are present in equal amounts is called a racemic mixture, or racemate. Racemic mixtures can be symbolized by a (d/l)- or ()- prefix in front of the substance's name. Since enantiomers have equal and opposite specific rotations, a racemic mixture exhibits no optical activity. Therefore it is impossible to tell a racemic mixture apart from an achiral substance using polarimetry alone. Note that the terms chiral and optically active should not be confused. It would be incorrect to say that a racemic mixture is achiral. Chirality is a property of individual molecules. Optical activity is a property of solutions. A racemic mixture consists of chiral molecules, but it has no net optical activity.
The process by which a racemic mixture is formed from chiral materials is called racemization. One way to do this is to mix equal amounts of enantiomeric substances. From this point of view, it may be puzzling that racemic mixtures are important. After all, what are the chances of obtaining any mixture in which two enantiomers are present in exactly equal amounts? It turns out that racemic mixtures actually occur with considerable frequency. Racemic mixtures are often formed when achiral substances are converted into chiral ones. This is due to the fact that chirality can only be distinguished in a chiral environment. An achiral substance in an achiral environment has no preference to form one enantiomer over another.
The separation of enantiomers poses a special problem for chemists. Enantiomers have the same boiling points, melting points, solubilities, etc., so many of the techniques used to separate other compounds don't work on racemic mixtures. The answer to this problem is to separatee nantiomers in a chiral environment, where they interact differently.
One technique is to use a chiral resolving agent. This technique relies on the fact that while enantiomers have identical physical properties, diastereomers generally have different properties. For example, suppose we wanted to separate the enantiomers of 2-hydroxylpropionic acid. We add as the resolving agent an enantiomerically pure amount of (R)-2-phenyl-ethylamine. The two enantiomers interact with (R)-2-phenyl-ethylamine to form two distinct salt species that are diastereomers of each other. The diastereomers can then be crystallized separately.
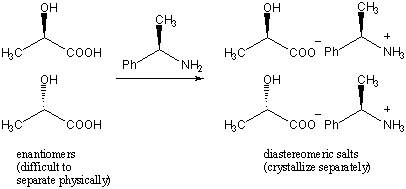
Another technique is to use chiral chromatography. In this process, the racemate is run through a column that is filled with a chiral substance. The enantiomers will interact differently with the substance and will then elute (or filter through the substance) at different rates. These techniques are also applied to mixtures of enantiomers beside racemic mixtures, for example to purify a species from small amounts of its enantiomer.
How important is it for chemists to isolate pure enantiomers? In some applications, the chirality of a molecule is unimportant. In many cases, however, the chirality of a molecule is crucial to its function. This is especially true in biological systems, where a molecule might have a function vastly different from that of its enantiomer. Biological systems are chiral environments. Here are a few examples:
Please wait while we process your payment





