Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Problems 1
Problem :
Suppose that we have a fuel cell battery, in which electrons flow from a terminal in one half cell to a terminal in another. Explain this phenomenon in terms of the chemical potential.
We can look at the battery as two systems in diffusive contact through the connecting wire. The electrons simply then flow from the cell with the higher chemical potential to that with the lower until an equilibrium is reached, if ever.
Problem :
Show that the units of pressure as we have defined it agree with those of the conventional understanding of pressure.
The conventional units are 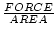 . We have defined
pressure so that we have an energy in the numerator and a volume in the
denominator. But remember that energy has the same units as work,
namely FORCE×LENGTH, and therefore we have
. We have defined
pressure so that we have an energy in the numerator and a volume in the
denominator. But remember that energy has the same units as work,
namely FORCE×LENGTH, and therefore we have
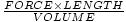 =
= 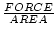 .
.
Problem :
Forcing a system into a small volume makes the energy of the system grow, whereas expanding the system, colloquially speaking, gives the particles more room to relax, and the energy of the system decreases (all for a process at constant entropy). Using the definition of pressure we've investigated, show what happens to the pressure at large volumes and very small volumes of the system. Does this agree with your intuition?
For a system of small volume for the number of particles, the energy is high. Increasing the volume some small amount, δV, will cause a great decrease in the energy U. Therefore, the pressure is:
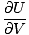 )σ = -
)σ = - 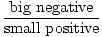 = big positive
= big positive
For a system of great volume for the number of particles, the energy is already low. Increasing the volume some small amount, δV, will only cause a small decrease in the energy U. Therefore, the pressure is:
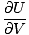 )σ = -
)σ = - 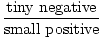 = small positive
= small positive
This makes sense to us. We expect a cramped system to have a high pressure and sprawling system to have low pressure.
Problem :
Is the energy of the system U an intensive or extensive variable?
Doubling the system should double the energy, so U is an extensive variable.
Problem :
Explain why the entropy is an extensive variable.
Remember that entropy was defined as σ = log g where g was the multiplicity function. We defined the entropy in this manner so that the entropies of two systems in contact would add together, since their individual g functions multiply together. So, doubling the system means that σnew = σoriginal + σduplicate = 2σoriginal. Therefore entropy is an extensive variable.
Please wait while we process your payment





