Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



Create Account
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Log into your PLUS account
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
Select Your Plan
Monthly
$5.99
/month + tax
Annual
$29.99
/year + taxAnnual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
Monthly
$5.99
/month + taxYou could save over 50%
by choosing an Annual Plan!

Annual
$29.99
/year + taxSAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
Annual
$22.49/month + tax
Save 25%
on 2-49 accounts
Annual
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
Testimonials from SparkNotes Customers
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Testimonials from SparkNotes Customers
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
Payment Information
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Thank You!
Your group members can use the joining link below to redeem their membership. They will be prompted to log into an existing account or to create a new account. All members under 16 will be required to obtain a parent's consent sent via link in an email.Your Child’s Free Trial Starts Now!
Thank you for completing the sign-up process. Your child’s SparkNotes PLUS login credentials are [email] and the associated password. If you have any questions, please visit our help center.Your Free Trial Starts Now!
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment
Month
Day
Year
Please read our terms and privacy policy
Please wait while we process your payment

Molecular Orbital Theory
To demonstrate why it is important to take the number of antibonding electrons into account in our bond order calculation, let us consider the possibility of making a molecule of He2. An orbital correlation diagram for He2 is provided in :
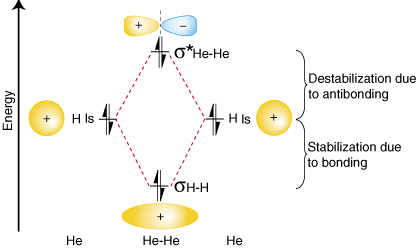
From the orbital correlation diagram above you should notice that the amount of stabilization due to bonding is equal to the amount of destabilization due to antibonding, because there are two electrons in the bonding orbital and two electrons in the antibonding orbital. Therefore, there is no net stabilization due to bonding so the He2 molecule will not exist. The bond order calculation shows that there will be a bond order of zero for the He2 molecule--exactly what we should predict given that helium is a noble gas and does not form covalent compounds.
Both hydrogen and helium only have 1s atomic orbitals so they produce very simple correlation diagrams. However, we have already developed the techniques necessary to draw a correlation diagram for a more complex homonuclear diatomic like diboron, B2. Before we can draw a correlation diagram for B2, we must first find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then, we rank them in order of increasing energy. Each boron atom has one 2s and three 2p valence orbitals. Due to the great difference in energy between the 2s and 2p orbitals, we can ignore the overlap of these orbitals with each other. All orbitals composed primarily of the 2s orbitals will be lower in energy than those comprised of the 2p orbitals. shows the process of creating the molecular orbitals for diboron by combining orbitals of atomic boron. Note that the orbitals of lowest energy have the most constructive overlap (fewest nodes) and the orbitals with the highest energy have the most destructive overlap (most nodes).
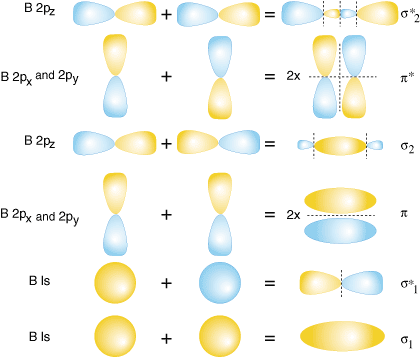
Notice that there are two different kinds of overlap for p-orbitals--end-on and side-on types of overlap. For the p-orbitals, there is one end-on overlap possible which occurs between the two pz. Two side-on overlaps are possible--one between the two px and one between the two p y. P-orbitals overlapping end-on create s bonds. When p-orbitals bond in a side-on fashion, they create p bonds. The difference between a p bond and a s bond is the symmetry of the molecular orbital produced. s bonds are cylindrically symmetric about the bonding axis, the z-direction. That means one can rotate the s bond about the z-axis and the bond remains the same. In contrast, p bonds lack that cylindrical symmetry and have a node passing through the bonding axis.
Now that we have determined the energy levels for B2, let's draw the orbital correlation diagram ():
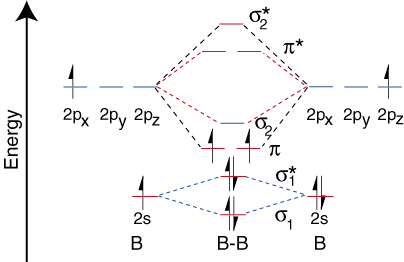
The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. It turns out that only when the bond lengths are relatively short (as in B2, C2, and N2) can the two p-orbitals on the bonded atoms efficiently overlap to form a strong p bond. Some textbooks explain this observation in terms of a concept called s-p mixing. For any atom with an atomic number greater than seven, the p bond is less stable and higher in energy than is the s bond formed by the two end-on overlapping p orbitals. Therefore, the following orbital correlation diagram for fluorine is representative of all homonuclear diatomic molecules with atomic numbers greater than seven.
Please wait while we process your payment





