Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!

Annual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
You could save over 50%
by choosing an Annual Plan!

SAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
$22.49/month + tax
Save 25%
on 2-49 accounts
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Colligative Properties
As you may have noticed when we looked at the , the freezing point is depressed due to the vapor pressure lowering phenomenon. The points out that fact:
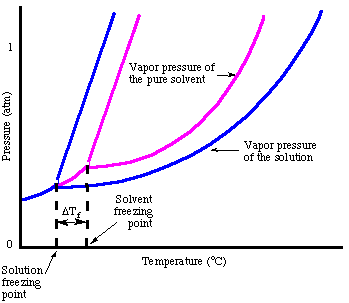
In analogy to the boiling point elevation, we can calculate the amount of the freezing point depression with the :
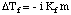
Note that the sign of the change in freezing point is negative because the freezing point of the solution is less than that of the pure solvent. Just as we did for boiling point elevation, we use molality to measure the concentration of the solute because it is temperature independent. Do not forget about the van't Hoff factor, i, in your freezing point calculations.
One way to rationalize the freezing point depression phenomenon without talking about Raoult's law is to consider the freezing process. In order for a liquid to freeze it must achieve a very ordered state that results in the formation of a crystal. If there are impurities in the liquid, i.e. solutes, the liquid is inherently less ordered. Therefore, a solution is more difficult to freeze than the pure solvent so a lower temperature is required to freeze the liquid.
Osmosis refers to the flow of solvent molecules past a semipermeable membrane that stops the flow of solute molecules only. When a solution and the pure solvent used in making that solution are placed on either side of a semipermeable membrane, it is found that more solvent molecules flow out of the pure solvent side of the membrane than solvent flows into the pure solvent from the solution side of the membrane. That flow of solvent from the pure solvent side makes the volume of the solution rise. When the height difference between the two sides becomes large enough, the net flow through the membrane ceases due to the extra pressure exerted by the excess height of the solution chamber. Converting that height of solvent into units of pressure (by using the ) gives a measure of the osmotic pressure exerted on the solution by the pure solvent. P stands for pressure, r is the density of the solution, and h is the height of the solution.
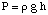
shows a typical setup for measuring the osmotic pressure of a solution.
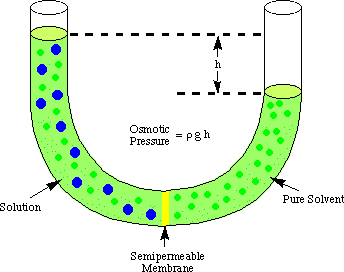
You can understand why more molecules flow from the solvent chamber to the solution chamber in analogy to our discussion of Raoult's law. More solvent molecules are at the membrane interface on the solvent side of the membrane than on the solution side. Therefore, it is more likely that a solvent molecule will pass from the solvent side to the solution side than vice versa. That difference in flow rate causes the solution volume to rise. As the solution rises, by the pressure depth equation, it exerts a larger pressure on the membrane's surface. As that pressure rises, it forces more solvent molecules to flow from the solution side to the solvent side. When the flow from both sides of the membrane are equal, the solution height stops rising and remains at a height reflecting the osmotic pressure of the solution.
The equation relating the osmotic pressure of a solution to its concentration has a form quite similar to the ideal gas law:
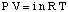
Although the above equation may be more simple to remember, the is more useful. This form of the equation has been derived by realizing that n / V gives the concentration of the solute in units of molarity, M.
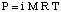
Please wait while we process your payment





