Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews July 18, 2025 July 11, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Solution Composition
A solution is a homogeneous mixture. That means the components of a solution are so evenly spread throughout the mixture that there are no perceivable differences in composition. Solutions can be formed by mixing two substances together such as sugar and water. If you pour a packet of sugar into a glass of water, initially you have a suspension as the sugar crystals float about in the glass. When you have stirred the sugar and water for long enough, you will eventually get a clear, colorless mixture. Some people, especially young children, can be fooled by such a demonstration into thinking that the sugar has "disappeared". However, as chemists, we know better. The law of conservation of matter states that the sugar can not just disappear, it must have gone somewhere else. That somewhere else is into solution. The sugar has become evenly dispersed. In fact the sugar molecules are so well spread out that we can no longer see a single sugar crystals. However, if you taste the water, you will find it to be sugary--confirming the presence of sugar in the water. The minor component of the solution is called the solute. In the present example, sugar is the solute. The major component of the solution is called the solvent. In this case water is the solvent.
Solutions can also be formed by mixing together many different phases of matter. For instance, air is a solution. The solute gasses oxygen, carbon dioxide, argon, ozone, and others are dissolved in the solvent nitrogen gas. Another example is found in "gold" jewelry. Most of the golden jewelry sold in the world is not 24 karat (i.e. 100% pure gold) but rather it is a solution of other metals, commonly silver and copper, in a gold solvent. Such a solution of metal(s) in another metal is called an amalgam.
Perhaps the most important property of a solution is its concentration. A dilute acetic acid solution, also called vinegar, is used in cooking while a concentrated solution of acetic acid would kill you if ingested. The only difference between such solutions is the concentration of the solute. In order to quantify the concentrations of solutions, chemists have devised many different units of concentration each of which is useful for different purposes.
Molarity, the number of moles of solute per liter of solution, has the units moles / L which are abbreviated M. This unit is the most commonly used measure of concentration. It is useful when you would like to know the number of moles of solute when you know both the molarity and the volume of a solution. For example, it is easy to calculate the volume of a 1.5 M solution of HCl necessary to completely react with 0.32 moles of NaOH:
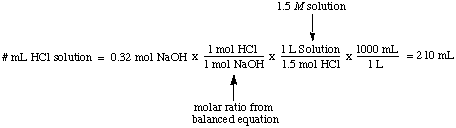
Normality, the number of molar equivalents of solute per liter of solution, has the units equivalents / L which are abbreviated N. To illustrate the difference between molarity and normality let's assume that we had used a 1.5 M solution of sulfuric acid, H2SO4, instead of a 1.5 M solution of hydrochloric acid, HCl in the above example. Because sulfuric acid can donate two protons to the NaOH, as noted in the , it will only take half as much sulfuric acid as hydrochloric acid to neutralize the sodium hydroxide.
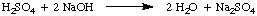
In the present example, the 1.5 M solution of sulfuric acid reacts like a 3.0 M solution of hydrochloric acid because there are two equivalents of H+ per mole of sulfuric acid. Therefore, that solution of sulfuric acid is 3.0 N.
Please wait while we process your payment

