Problem : CaCO3(s) + 176 kJ→CaO(s) + CO2(g)
How much energy is required to decompose 10.4 moles of
CaCO3(s)?
10.4 moles×176 kJ / mole = 1830 kJ
Problem : SO3(g) + H2O(l)→H2SO4(aq) + 129.6 kJ
At STP, if 540 kJ is released, what volume of SO3(g) was
originally added?
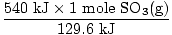 | = | 4.17 moles SO3(g) |
|
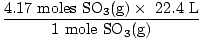 | = | 93.3 L |
|