Besides their active sites, many enzymes have other locations or crevices where molecules can bind. Regulatory sites, also called allosteric sites, are places other than the active site of the enzyme that serve to regulate enzymatic activity.
Allosteric sites as inhibitors
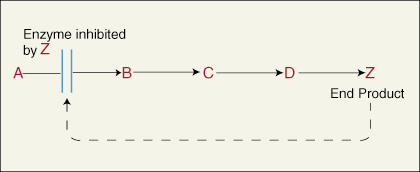
The end product in a series of reactions catalyzed by different enzymes at each
step may bind to the allosteric site and inhibit the activity of the first
enzyme in the pathway. When an inhibitory molecule binds to an allosteric
region on the enzyme it can cause the active site of the enzyme to close up and
become inactive.
Allosteric sites as stimulators
Allosteric sites can also be areas that cause the stimulation of enzymatic rates. When these allosteric sites are occupied, the enzyme's active site may change in shape, becoming more efficient or receptive as a catalyst.
Covalent Modification
Further regulation of enzymes comes in the form of covalent modification. Many enzymes are regulated by reversible attachment of phosphoryl groups to serine andthreonine amino acid residues. Specific types of enzymes called kinases phosphorylate add phosphoryl groups to other enzymes while phosphatases remove phosphate groups. By adding just one covalent bond to the enzyme, its activity can be drastically changed. For example, during levels of low blood glucose, glucagon and epinephrine are secreted into the blood and bind to muscle and brain cell receptors. Upon binding, these hormones cause a cascade of effects within the cell, which results in the phosphorylation of a number of proteins, including a series of enzymes involved in metabolism. All phosphorylations act to increase the rate of enzymes involved in glycogen and triglyceride degradation while inhibiting the rate of enzymes involved in glycolysis and the Citric Acid Cycle. In effect, the addition of phosphate groups to these enzymes causes the level of blood glucose to increase.
Membrane channels and pumps
Proteins are also abundant within biological membranes. Many cellular receptors, channels, and pumps are bound to membranes. Since these proteins span across a nonpolar environment, many of their residues facing this environment are also nonpolar, allowing more favorable interactions to occur. Both channels and pumps are involved in the regulation of fluids and ions within and outside the cell. However, they differ in many key respects. Channels allow ions to flow from an area of high concentration to an area of low concentration. This is a completely passive process. Pumps, on the other hand, force ions up their concentration gradient from a region of lower concentration to a region of higher concentration. This process is called active transport and usually requires the energy of adenosine triphosphate (ATP) in order to overcome the energy barrier.
A classic example of a pump is the sodium potassium pump. Since during the excitation of neurons sodium ions are constantly traversing into the cell and potassium ions are leaving the cell, the resting levels of these ions must be constantly restored. Unlike potassium, which is present in larger proportions outside the cell, sodium is in much higher concentrations outside the cell. The sodium potassium pump pushes sodium and potassium against their concentration gradients by binding ATP and hyrolyzing it for energy.
Immune function
Proteins are also important in the immune response. There are two types of important proteins the immune system uses to scan the vast network of molecules in our body and determine self from nonself. In the first stage of the immune response, the body recognizes circulating foreign particles (antigens) through antibodies, which are produced by plasma cells (B lymphocytes). The ability of plasma cells to produce millions of different antibodies is inherent from birth; virtually any circulating antigen will be bound by its complementary antibody. Once bound by antibody, the antigen can be either consumed by macrophages or be further bound by mature plasma cells to stimulate the production of even more antibodies. Mature cells are stimulated to divide and form clones, which act as an immunological memory against further attack from identical antigens. Like enzymes, the amino acid sequence of the antibody determines its specificity. The whole recognition and response of plasma cells to a new infection is called the humoral response.
The second stage of immune response is called the cellular immune response. In the cellular response, T lymphocytes (killer T cells) bind to foreign particles displayed on the surface of cells and destroy the contaminated cell. Helper T cells also bind to the foreign particles displayed on the surface of cells and stimulate the humoral response by helping plasma cells to proliferate. Why is the cellular response necessary if antibodies can recognize antigens and mark them for destruction? The answer lies in the fact that many of the viruses and bacteria that invade the body are found in greater concentrations within cells, preventing antibodies from reaching them. The immune system has adapted to this problem by cutting some of the foreign particles up into peptides to be displayed at the surface of the infected cell by a protein known as the major histocompatibility complex (MHC). Killer and helper T cells are specialized for recognizing peptides bound to these proteins, increasing the speed and effectiveness of the immune response.


 payment page
payment page



