The Entropy of an Ideal Gas
We use the relation σ = - 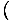
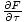
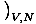 to find the entropy from the free energy. Without much work, we come up
with:
to find the entropy from the free energy. Without much work, we come up
with:
The Energy of an Ideal Gas
Remember that the free energy can be defined in terms of the energy as
follows: F = U - τσ. We rearrange to solve for U, and plug in
our values for F and σ to find the simple result:
U =
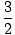 Nτ
Nτ
The Heat Capacity of an Ideal Gas
A measure of how much heat a gas can hold is the heat capacity.
There are two slightly different measures of the heat capacity. One,
the heat capacity at constant volume, is defined as
CVâÉá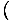
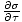
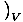 . The other, the heat capacity at constant pressure, is defined as
CpâÉá
. The other, the heat capacity at constant pressure, is defined as
CpâÉá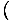
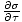
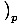 .
.
The only difference between the two definitions is in what is held
constant in the derivative. The results for an ideal gas can be
obtained by direct substitution and differentiation for the heat
capacity at constant volume, and by the thermodynamic identity for the
heat capacity at constant pressure. The results are:
CV =
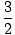 N
N
Cp =
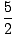 N
N
Remember that these are in fundamental
units, and we need to multiply by the Boltzmann constant kB to change
to conventional units.
We define the ratio of the two heat capacities, Cp/CV, to be
γ. For an ideal gas, γ = 5/3.
Equipartition
There is a good shortcut to find the energy of any classical system,
known as equipartition. The theory states that every particle has
energy equal to 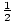 τ for each degree of freedom of the
particle, which can be gleaned from the number of quadratic terms in the
expression for the energy.
τ for each degree of freedom of the
particle, which can be gleaned from the number of quadratic terms in the
expression for the energy.
Let us make the theory clearer by applying it to the ideal gas. Each
particle in the ideal gas has classical energy equal to
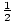 mv2. Here, the velocity is a vector having 3 components. In
Cartesian, there are vx, vy, and vz. Therefore each particle
has energy
mv2. Here, the velocity is a vector having 3 components. In
Cartesian, there are vx, vy, and vz. Therefore each particle
has energy 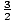 τ. Summing up for all N particles in the
system gives the same answer we got before, U =
τ. Summing up for all N particles in the
system gives the same answer we got before, U = 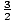 Nτ.
Nτ.