Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews July 8, 2025 July 1, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Buffered Solutions
As you have seen in calculating the pH of solutions, only a small amount of a strong acid is necessary to drastically alter the pH. For certain experiments, however, it is desirable to keep a fairly constant pH while acids or bases are added to the solution either by reaction or by the experimenter. Buffers are designed to fill that role. Chemists use buffers routinely to moderate the pH of a reaction. Biology finds manifold uses for buffers which range from controlling blood pH to ensuring that urine does not reach painfully acidic levels.
A buffer is simply a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers work by reacting with any added acid or base to control the pH. For example, let's consider the action of a buffer composed of the weak base ammonia, NH3, and its conjugate acid, NH4+. When HCl is added to that buffer, the NH3 "soaks up" the acid's proton to become NH4+. Because that proton is locked up in the ammonium ion, it proton does not serve to significantly increase the pH of the solution. When NaOH is added to the same buffer, the ammonium ion donates a proton to the base to become ammonia and water. Here the buffer also serves to neutralize the base.
As the above example shows, a buffer works by replacing a strong acid or base with a weak one. The strong acid's proton is replaced by ammonium ion, a weak acid. The strong base OH- was replaced by the weak base ammonia. These replacements of strong acids and bases for weaker ones give buffers their extraordinary ability to moderate pH.
Buffers must be chosen for the appropriate pH range that they are called on to control. The pH range of a buffered solution is given by the Henderson- Hasselbalch equation. For the purpose of the derivation, we will imagine a buffer composed of an acid, HA, and its conjugate base, A-. We know that the acid dissociation constant pKa of the acid is given by this expression:

The equation can be rearranged as follows:
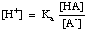
Taking the -log of this expression and rearranging the terms to make each one positive gives the Henderson-Hasselbalch equation:
Please wait while we process your payment

