Sn2 Rate and Transition State
The SN2 reaction is one of the most common in organic chemistry.
| CH3O- + CH3Br→CH3OCH3 + Br- |
The reaction of methoxide ion with methyl bromide proceeds via an SN2 mechanism. It has a second order rate law:
| rate = k [CH3O-] [CH3Br] |
The reaction is first order with regards to methoxide ion and methyl bromide, and second order overall. This means that the transition state must involve one molecule of methoxide and one molecule of methyl bromide. The sim plest possible transition state is one where the methoxide nucleophile substitutes for the bromide ion:
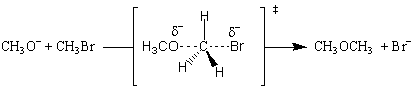
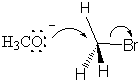
The transition state is formed by the flow of electrons from the nucleophilic methoxide ion into the C-O bond. At the same time, the electrons from the C-Br bond flow onto the bromine leaving group, weaken the bond and place a partial negative charge onto the bromine atom. All of this happens simultaneously, and the reaction is said to be concerted. All SN2 mechanisms are concerted.
SN2 Stereochemistry
The rate law indicates which molecules are in the transition state, but it does not specify how they come
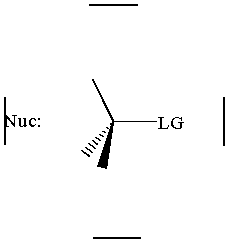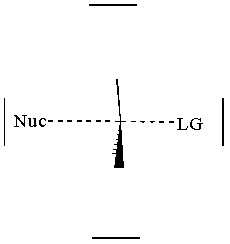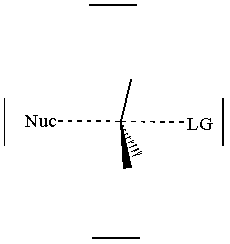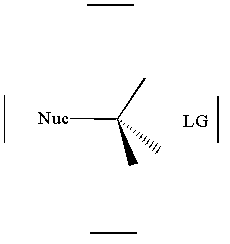
together. This can be accomplished by attacking a stereocenter:
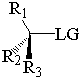
The nucleophile can attack the stereocenter in two ways. In frontside attack, it attacks from the same side as the leaving group. In backside attack, it attacks from the opposite side of the leaving group. These two modes of attack give retenti
on and inversion of stereochemical configuration, respectively. Retention and inversion will yield two different stereoisomers.
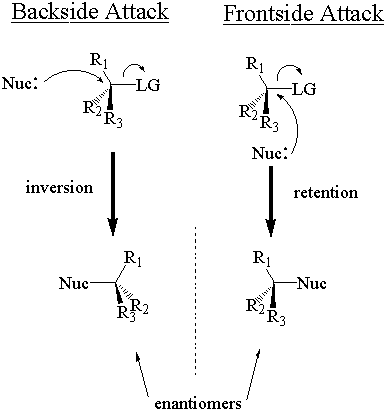
The phrase "inversion of configuration" may lead you to believe that the absolute configuration must switch after SN2 attack. This is not always true. Recall that absolute stereochemical configuration is determined via the Cahn-Ingold-Prelog (CIP) system, and categorized with the labels "R" and "S." The absolute configuration inverts (from "R" to "S" or vice-versa) only when the nucleophile and leaving group have the same CIP priority relative to the other substituents. Since good nucleophiles and leaving gro ups tend towards equally high CIP priorities, most SN2 reactions do result in a switch of absolute configuration. If the nucleophile and leaving group have different relative CIP priorities, however, the absolute configuration does not necessarily change even though inversion occurs. Keep on your toes.
A favorite test question presents a SN2 reaction that results in 100% retention of both absolute and general configuration. In this case, it's likely that twoSN2 reactions take place. Two inversions yield a net retention of configuration.
Molecular Orbital Explanation of SN2
The stereospecificity of the SN2 immediately begs the question of why it must proceed through backside attack. There are two common explanations:
- The steric argument states that there's simply more room for the nucleophile to attack from the back. If the nucleophile attacked from the front, it would collide with the leaving group. This steric clash makes front side attack impossible.
- The molecular orbital hinges on the σ* carbon-leaving group antibond.
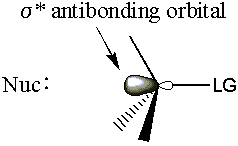
Recall from molecular orbital bonding theory that electrons donated into an antibondg orbital weaken the corresponding bond. So as the nucleophile donates electron density into the σ* C-LG antibond, the σ C-LG bond will weaken. The σ C-LG bond must break for the leaving group to leave. Since the σ* C-LG antibond is very accessible from the back, MO theory gives a ti dy explanation for backside attack.


 payment page
payment page



