E2 Rate and Transition State
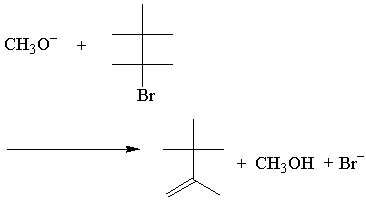
The rate law of the above E2 reaction follows:
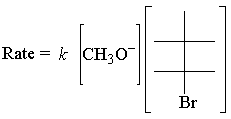
The E2 rate law is first order for both reactants. Here's the simplest possible transition
state:
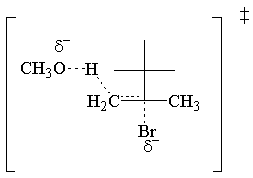
E2 Stereochemistry
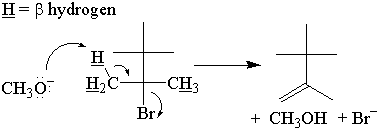
As in the SN2 reaction, the mechanism of transition state formation can be deduced using a stereocenter. There are two possible modes of reactivity that yield two different stereoisomers.
In syn elimination, the base attacks the β-hydrogen on the same side as the leaving group.
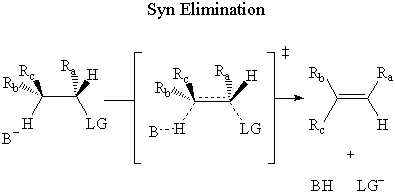
In anti elimination, the base attacks the β-hydrogen on the opposite side of the
leaving group.
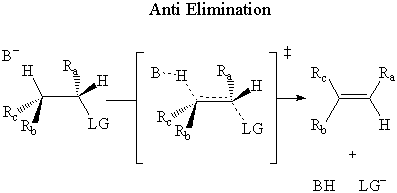
It has been experimentally determined that E2 elimination occurs through an anti mechanism.
Steric and Molecular Orbital Explanation of Anti Elimination
Just as with the SN2 backside attack mechanism, there are steric and molecular orbital explanations for E2 anti elimination.
The steric argument notes that the syn transition state is an eclipsed conformation. The anti state is in the more energetically stable staggered conformation. Since the rate of a reaction is inversely proportional to the stability of the transition state, the anti route should be much more favorable.
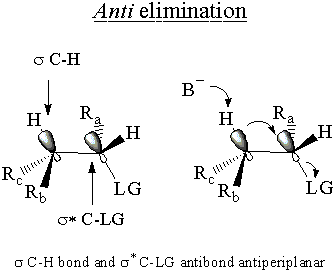
The E2molecular orbital argument hinges on the σ* C-LG antibond, just like
the SN2 MO argument. In this case, the σ* C-LG antibond is only
accessible to the electrons of the σ C-H bond if th e two bonds are antiperiplanar. In other
words the C-H and C-LG bonds must point opposite directions in parallel planes. If this condition is
met, the electrons of the σ C-H bond donate into the σ* C-LG antibond.
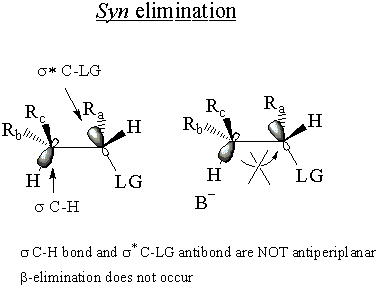
In the syn elimination, the bonds are not antiperiplanar, and the electrons of the σ
C-H bond cannot attack the σ* C-LG antibond. Thus E2 cannot occur through
syn elimination.
Saytzeff's Rule
Recall the first E2 reaction presented in this section:

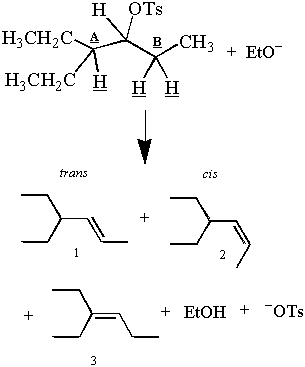
But wait! The observed products seem to contradict the antiperiplanar argument. There are three products and three β-hydrogens, but only two of those hydrogens may be antiperiplanar to the C-OTs bond at one time. How can the two hydrogens off carbon B give two products, even though only one can be antiperiplanar?
While only one hydrogen off carbon B can be antiperiplanar at one time, both hydrogens spend some time in the antiperiplanar position. Thus the elimination of the hydrogens off carbon B gives the cis and trans products. This is possible because alkanes that are not inordinately hindered rotate about their C-C bonds. For an example of a structure that cannot rotate freely about its C-C bond, see problem #4. A deeper discussion of C-C bond rotation can be found under conformational analysis.
Product 3 is the major product because an E2 elimination favors the formation of the most stable alkene. The stability of an alkene is proportional to the degree of substitution at its double-bond carbons. The double-bonds of products 1 and 2 are less branched than the double-bonds of product 1. Thus there will be more of product 3 than products 1 and 2 after the reaction has run to completion. E2's preference for the more stable (and more highly branched) alkene is called Saytzeff's Rule.


 payment page
payment page



